Abstract
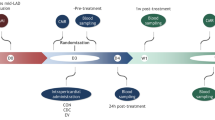
Sustainable and reproducible large animal models that closely replicate the clinical sequelae of myocardial infarction (MI) are important for the translation of basic science research into bedside medicine. Swine are well accepted by the scientific community for cardiovascular research, and they represent an established animal model for preclinical trials for US Food and Drug Administration (FDA) approval of novel therapies. Here we present a protocol for using porcine models of MI created with a closed-chest coronary artery occlusion-reperfusion technique. This creates a model of MI encompassing the anteroapical, lateral and septal walls of the left ventricle. This model infarction can be easily adapted to suit individual study design and enables the investigation of a variety of possible interventions. This model is therefore a useful tool for translational research into the pathophysiology of ventricular remodeling and is an ideal testing platform for novel biological approaches targeting regenerative medicine. This model can be created in approximately 8–10 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Heidenreich, P.A. et al. Forecasting the future of cardiovascular disease in the United States. Circulation 123, 933–944 (2011).
Roger, V.L. et al. Heart Disease and Stroke Statistics—2011 Update. Circulation 123, e18–e209 (2011).
Swindle, M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques (Taylor & Francis Group, 2007).
Dondelinger, R.F. et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur. Radiol. 8, 1254–1273 (1998).
Amado, L.C. et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 102, 11474–11479 (2005).
Hatzistergos, K.E. et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation/novelty and significance. Circ. Res. 107, 913–922 (2010).
Ishikawa, K. et al. Development of a preclinical model of ischemic cardiomyopathy in swine. Am. J. Physiol. Heart Circ. Physiol. 301, H530–H537 (2011).
Mewton, N. et al. Determination of the myocardial area at risk with pre- versus post-reperfusion imaging techniques in the pig model. Basic Res. Cardiol. 106, 1247–1257 (2011).
Schuleri, K.H. et al. The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp. Med. 58, 568–579 (2008).
Verdouw, P.D., van den Doel, M.A., de, Z.S. & Duncker, D.J. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc. Res. 39, 121–135 (1998).
Zhang, J. et al. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model: MRI and 31P-MRS Study. Circulation 94, 1089–1100 (1996).
Pacher, P., Nagayama, T., Mukhopadhyay, P., Batkai, S. & Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3, 1422–1434 (2008).
Kohn, F., Sharifi, A.R. & Simianer, H. Modeling the growth of the Goettingen minipig. J. Animal Sci. 85, 84–92 (2007).
Suzuki, Y., Lyons, J.K., Yeung, A.C. & Ikeno, F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Cathet. Cardiovasc. Intervent. 71, 100–107 (2008).
Pfeffer, M.A. & Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81, 1161–1172 (1990).
Williams, A.R. & Hare, J.M. Mesenchymal stem cells; biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 109, 923–940 (2011).
Hou, D. et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery. Circulation 112, 150–156 (2005).
Perin, E.C. et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J. Mol. Cellular Cardiol. 44, 486–495 (2008).
Freyman, T. et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 27, 1114–1122 (2006).
Wang, X. et al. Stem cells for myocardial repair with use of a transarterial catheter. Circulation 120, S238–S246 (2009).
Trachtenberg, B. et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am. Heart J. 161, 487–493 (2011).
Quevedo, H.C. et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 106, 14022–14027 (2009).
Bolli, R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011).
Williams, A.R. et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy/novelty and significance. Circ. Res. 108, 792–796 (2011).
Huang, N.F. et al. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat. Protoc. 1, 1596–1609 (2006).
Kornowski, R. et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy: results in normal and ischemic porcine models. J. Am. Coll. Cardiol. 35, 1031–1039 (2000).
van Laake, L.W. et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat. Protoc. 2, 2551–2567 (2007).
Suncion, V.Y., Schulman, I.H. & Hare, J.M. Concise review: the role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Trans. Med. 1, 29–35 (2012).
Vilahur, G. et al. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 50, 522–533 (2011).
de la Fuente, L.M. et al. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the TABMMI study). Am. Heart J. 154, 79 (2007).
Tomkowiak, M.T. et al. Targeted transendocardial therapeutic delivery guided by MRI-X-ray image fusion. Catheter. Cardiovasc. Interv. 78, 468–478 (2011).
Baklanov, D.V. et al. Live 3D echo guidance of catheter-based endomyocardial injection. Catheter. Cardiovasc. Interv. 65, 340–345 (2005).
Fuchs, S. et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J. Am. Coll. Cardiol. 37, 1726–1732 (2001).
Perin, E.C. et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 107, 2294–2302 (2003).
Dib, N. et al. Endoventricular transplantation of allogenic skeletal myoblasts in a porcine model of myocardial infarction. J. Endovasc. Ther. 9, 313–319 (2002).
Losordo, D.W. et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina. Circulation 115, 3165–3172 (2007).
van Ramshorst, J. et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia. JAMA. 301, 1997–2004 (2009).
Krause, K. et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart 95, 1145–1152 (2009).
Smits, P.C. et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J. Am. Coll. Cardiol. 42, 2063–2069 (2003).
Dib, N. et al. Safety and feasibility of autologous myoblast transplantation in patients With ischemic cardiomyopathy. Circulation 112, 1748–1755 (2005).
Ding, P. et al. Horner syndrome after carotid sheath surgery in a pig: anatomic study of cervical sympathetic chain. Comp. Med. 61, 453–456 (2011).
Scanlon, P.J. et al. ACC/AHA guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. J. Am. Coll. Cardiol. 33, 1756–1824 (1999).
Alderman, E.L. & Stadius, M. The angiographic definitions of the bypass angioplasty revascularization investigation. Coron. Artery Dis. 3, 1189–1207 (1992).
Acknowledgements
This research is supported by US National Institutes of Health grants R01 HL094849, P20 HL101443, R01 HL084275, R01 HL107110, R01 HL110737 and U54 HL081028 (J.M.H.); and by the University of Miami Miller School of Medicine, Interdisciplinary Stem Cell Institute. The researchers would like to thank R. Gonzalez, D. Valdes, J. Rodriguez and M. Rosado for their technical contributions, and C. Sanina for her artistic contributions.
Author information
Authors and Affiliations
Contributions
F.C.M. was involved in manuscript conception and design, creation/collection/assembly of data and manuscript writing; K.S.T. contributed to collection/assembly of data and manuscript writing; V.K. and V.Y.S. contributed equally to manuscript writing and editing, and to the creation and collection of data; A.W.H. and A.R.W. contributed to surgical and experimental conception and design, and to manuscript editing; M.M. was involved in manuscript writing; J.M.H., team leader, provided project and manuscript conception and design, financial support, manuscript writing and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Hare has a research grant from BioCardia and is listed as an inventor on a patent for cell therapy filed by the University of Miami. No other authors indicate competing financial interests.
Rights and permissions
About this article
Cite this article
McCall, F., Telukuntla, K., Karantalis, V. et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc 7, 1479–1496 (2012). https://doi.org/10.1038/nprot.2012.075
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2012.075
This article is cited by
-
Biologically derived epicardial patch induces macrophage mediated pathophysiologic repair in chronically infarcted swine hearts
Communications Biology (2023)
-
Combination human umbilical cord perivascular and endothelial colony forming cell therapy for ischemic cardiac injury
npj Regenerative Medicine (2023)
-
Free-breathing gradient recalled echo-based CMR in a swine heart failure model
Scientific Reports (2022)
-
A Robust Percutaneous Myocardial Infarction Model in Pigs and Its Effect on Left Ventricular Function
Journal of Cardiovascular Translational Research (2021)
-
The use of large animals to facilitate the process of MSC going from laboratory to patient—‘bench to bedside’
Cell Biology and Toxicology (2020)