Abstract
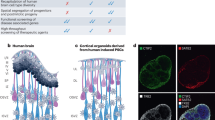
Human brain development exhibits several unique aspects, such as increased complexity and expansion of neuronal output, that have proven difficult to study in model organisms. As a result, in vitro approaches to model human brain development and disease are an intense area of research. Here we describe a recently established protocol for generating 3D brain tissue, so-called cerebral organoids, which closely mimics the endogenous developmental program. This method can easily be implemented in a standard tissue culture room and can give rise to developing cerebral cortex, ventral telencephalon, choroid plexus and retinal identities, among others, within 1–2 months. This straightforward protocol can be applied to developmental studies, as well as to the study of a variety of human brain diseases. Furthermore, as organoids can be maintained for more than 1 year in long-term culture, they also have the potential to model later events such as neuronal maturation and survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lanza, R., Gearhart, J., Hogan, B., Melton, D. & Pedersen, R. Essentials of Stem Cell Biology (Elsevier, 2009).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Sasai, Y., Eiraku, M. & Suga, H. In vitro organogenesis in three dimensions: self-organising stem cells. Development 139, 4111–4121 (2012).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Antonica, F. et al. Generation of functional thyroid from embryonic stem cells. Nature 491, 66–71 (2012).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Suga, H. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011).
Koehler, K.R., Mikosz, A.M., Molosh, A.I., Patel, D. & Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013).
Xia, Y. et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 15, 1507–1515 (2013).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Spence, J.R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Humphreys, B.D. Kidney structures differentiated from stem cells. Nat. Cell Biol. 16, 19–21 (2013).
Gilbert, S.F. Developmental Biology (Sinauer Associates, 2000).
Evans, M. Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat. Rev. Mol. Cell Biol. 12, 680–686 (2011).
Shevde, N.K. & Mael, A.A. Techniques in embryoid body formation from human pluripotent stem cells. Methods Mol. Biol. 946, 535–546 (2013).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Zhang, S.C., Wernig, M., Duncan, I.D., Brüstle, O. & Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Hu, B.-Y. & Zhang, S.-C. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol. Biol. 636, 123–137 (2010).
Wu, Y., Liu, Y., Chesnut, J.D. & Rao, M.S. Isolation of neural stem and precursor cells from rodent tissue. Methods Mol. Biol. 438, 39–53 (2008).
Price, P.J. & Brewer, G.J. Serum-free media for neural cell cultures. In Protocols for Neural Cell Culture (eds. Federoff, S. and Richardson, A.) 255–264 (Humana Press, 2001).
Elkabetz, Y. et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165 (2008).
Gaspard, N. et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357 (2008).
Brewer, G.J., Torricelli, J.R., Evege, E.K. & Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35, 567–576 (1993).
Koch, P., Opitz, T., Steinbeck, J.A., Ladewig, J. & Brüstle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA 106, 3225–3230 (2009).
Siegenthaler, J.A. et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597–609 (2009).
Petros, T.J., Tyson, J.A. & Anderson, S.A. Pluripotent stem cells for the study of CNS development. Front. Mol. Neurosci. 4, 30 (2011).
Eiraku, M. & Sasai, Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 22, 768–777 (2012).
Nasu, M. et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE 7, e53024 (2012).
Li, M.L. et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl. Acad. Sci. USA 84, 136–140 (1987).
Martin, I., Wendt, D. & Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 22, 80–86 (2004).
Ranga, A., Gjorevski, N. & Lutolf, M.P. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 69–70, 19–28 (2014).
Conti, L. & Cattaneo, E. Neural stem cell systems: physiological players or in vitro entities? Nat. Rev. Neurosci. 11, 176–187 (2010).
Shi, Y., Kirwan, P., Smith, J., Robinson, H.P.C. & Livesey, F.J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 15, 477–86 S1 (2012).
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284–20289 (2013).
Lamonica, B.E., Lui, J.H., Hansen, D.V. & Kriegstein, A.R. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat. Commun. 4, 1665 (2013).
Gilmore, E.C. & Walsh, C.A. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip. Rev. Dev. Biol. 2, 461–478 (2013).
Brennand, K.J. & Gage, F.H. Modeling psychiatric disorders through reprogramming. Dis. Model Mech. 5, 26–32 (2012).
Koike, M., Kurosawa, H. & Amano, Y. A round-bottom 96-well polystyrene plate coated with 2-methacryloyloxyethyl phosphorylcholine as an effective tool for embryoid body formation. Cytotechnology 47, 3–10 (2005).
Acknowledgements
We are grateful to members of the Knoblich laboratory for their technical expertise and feedback, and particularly to M. Renner and A. Peer for their experimental support. We also thank the Stem Cell and BioOptics core facilities of IMBA and the Institute of Molecular Pathology for their technical support. M.A.L. received funding from a European Molecular Biology Organization (EMBO) postdoctoral fellowship, a Helen Hay Whitney postdoctoral fellowship and a Marie Curie International Incoming Fellowship. Work in J.A.K.'s laboratory is supported by the Austrian Academy of Sciences, the Austrian Science Fund (FWF; projects Z153-B09 and I552-B19) and an advanced grant from the European Research Council.
Author information
Authors and Affiliations
Contributions
J.A.K. and M.A.L. wrote the manuscript, and M.A.L. performed the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lancaster, M., Knoblich, J. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9, 2329–2340 (2014). https://doi.org/10.1038/nprot.2014.158
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2014.158
This article is cited by
-
CUL4B mutations impair human cortical neurogenesis through PP2A-dependent inhibition of AKT and ERK
Cell Death & Disease (2024)
-
Apically localized PANX1 impacts neuroepithelial expansion in human cerebral organoids
Cell Death Discovery (2024)
-
Cerebral organoids with chromosome 21 trisomy secrete Alzheimer’s disease-related soluble aggregates detectable by single-molecule-fluorescence and super-resolution microscopy
Molecular Psychiatry (2024)
-
Schizophrenia endothelial cells exhibit higher permeability and altered angiogenesis patterns in patient-derived organoids
Translational Psychiatry (2024)
-
Research progress and application of liver organoids for disease modeling and regenerative therapy
Journal of Molecular Medicine (2024)