Abstract
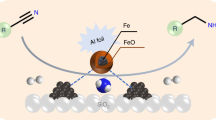
In this protocol, we describe the preparation of nanoscale iron oxide–based materials and their use in the catalysis of different hydrogenation reactions. Pyrolysis of a Fe(OAc)2-phenanthroline complex on carbon at 800 °C under argon atmosphere results in the formation of nanoscale Fe2O3 particles surrounded by nitrogen-doped graphene layers. By applying these catalysts, the hydrogenation of structurally diverse and functionalized nitroarenes to anilines proceeds with excellent selectivity. Furthermore, we have shown that one-pot reductive amination of carbonyl compounds with nitroarenes is also possible in the presence of these iron oxide catalysts. We report herein the synthesis of more than 40 amines, which are important feedstocks and key intermediates for pharmaceuticals, agrochemicals and polymers. The detailed preparation of the catalysts and the procedures for the hydrogenation processes are presented. The overall time required for the catalyst preparation and for the hydrogenation reactions are 35 h and 20–35 h, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
References
Rylander, P.N. Catalytic Hydrogenation in Organic Syntheses (Academic Press, 1979).
Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis. John Wiley & Sons, 2001.
Bullock, R.M. Abundant metals give precious hydrogenation performance. Science 342, 1054–1055 (2013).
Smith, A.M. & Whyman,, R. Review of methods for the catalytic hydrogenation of carboxamides. Chem. Rev. 114, 5477–5510 (2014).
Rylander, P.N. Catalytic Hydrogenation Over Platinum Metals (Academic Press, 1967).
Nishimura, S. Heterogeneous Catalytic Hydrogenation (John Wiley & Sons, 2001).
Platinum Today: Home-Johnson Matthey http://www.platinum.matthey.com.
Plietker, B. Iron Catalysis in Organic Chemistry (Wiley-VCH, 2008).
Bolm, C. A new iron age. Nat. Chem. 1, 420 (2009).
Bolm, C., Legros, J., Paih, J.L. & Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 104, 6217–6254 (2004).
Enthaler, S., Junge, K. & Beller, M. Sustainable metal catalysis with iron: from rust to a rising star. Angew. Chem. Int. Ed. Engl. 47, 3317–3321 (2008).
Sun, C.-L., Li, B.-J. & Shi, Z.-J. Direct CH transformation via iron catalysis. Chem. Rev. 111, 1293–1314 (2011).
Gopalaiah, K. Chiral iron catalysts for asymmetric synthesis. Chem. Rev. 113, 3248–3296 (2013).
Nakamura, E. & Sato, K. Managing the scarcity of chemical elements. Nat. Mater. 10, 158–161 (2011).
Marek, I. & Rappoport, Z. The Chemistry of Organoiron Compounds (John Wiley & Sons, 2013).
Ono, N. The Nitro Group in Organic Synthesis (Wiley-VCH, 2001).
Blaser, H.-U., Siegrist, U. & Steiner,, H. Aromatic nitro compounds: fine chemicals through heterogeneous catalysis. (Wiley-VCH, 2001).
Blaser, H.-U. & Steiner, H. Selective catalytic hydrogenation of functionalized nitroarenes: an update. ChemCatChem 1, 210–221 (2009).
Li, J., Shi, X.-Y., Bi, Y.-Y., Wei, J.-F. & Chen, Z.-G. Pd nanoparticles in ionic liquid brush: a highly active and reusable heterogeneous catalytic assembly for solvent-free or on-water hydrogenation of nitroarene under mild conditions. ACS Catal. 1, 657–664 (2011).
Takasaki, M. et al. Chemoselective hydrogenation of nitroarenes with carbon nanofiber-supported platinum and palladium nanoparticles. Org. Lett. 10, 1601–1604 (2008).
Motoyama, Y. et al. Platinum nanoparticles supported on nitrogen-doped carbon nanofibers as efficient poisoning catalysts for the hydrogenation of nitroarenes. ChemCatChem 3, 1578–1581 (2011).
Pandarus, V., Ciriminna, R., Beland, F. & Pagliaro, M. A new class of heterogeneous platinum catalysts for the chemoselective hydrogenation of nitroarenes. Adv. Synth. Catal. 353, 1306–1316 (2011).
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006).
Jagadeesh, R.V. et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 342, 1073–1076 (2013).
Li, L. et al. A PdAg bimetallic nanocatalyst for selective reductive amination of nitroarenes. Chem. Commun. 49, 6843–6845 (2013).
Tang, C.-H. et al. Direct one-pot reductive N-alkylation of nitroarenes by using alcohols with supported gold catalysts. Chem. Eur. J. 17, 7172–7177 (2011).
Sreedhar, B., Reddy, P.S. & Devi, D.K. Direct one-pot reductive amination of aldehydes with nitroarenes in a domino fashion: catalysis by gum-acacia-stabilized palladium nanoparticles. J. Org. Chem. 74, 8806–8809 (2009).
Stemmler, T., Surkus, A.-E., Junge, K. & Beller, M. Iron-catalyzed synthesis of secondary amines: on the way to green reductive aminations. ChemSusChem 7, 3012–3016 (2014).
Acknowledgements
The German Federal Ministry of Education and Research (BMBF) and the State of Mecklenburg-Vorpommern are gratefully acknowledged for their general support. We are grateful to A. Brückner, J. Radnik and M.-M. Pohl for the characterization of catalysts. We thank the analytical department of Leibniz-Institute for Catalysis, for their excellent service.
Author information
Authors and Affiliations
Contributions
R.V.J., T.S. and M.B. planned the project and wrote the paper. R.V.J. and T.S. developed and prepared the catalysts. A.-E.S., H.J., K.J. and M.B. were involved in the development of this class of catalysts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Data (PDF 5664 kb)
Rights and permissions
About this article
Cite this article
Jagadeesh, R., Stemmler, T., Surkus, AE. et al. Hydrogenation using iron oxide–based nanocatalysts for the synthesis of amines. Nat Protoc 10, 548–557 (2015). https://doi.org/10.1038/nprot.2015.025
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2015.025
This article is cited by
-
Functionalized nanoparticles and their environmental remediation potential: a review
Journal of Nanostructure in Chemistry (2022)
-
Copper nanoparticles (CuNPs) catalyzed chemoselective reduction of nitroarenes in aqueous medium
Journal of Chemical Sciences (2021)
-
Metallo-aerogels derived from chitosan with encapsulated metal nanoparticles as robust, efficient and selective nanocatalysts towards reduction of nitroarenes
Nano Research (2021)
-
Reductive amination using cobalt-based nanoparticles for synthesis of amines
Nature Protocols (2020)
-
Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines
Nature Communications (2018)


Bronwen Dekker
Editors note: The author has noticed that on page 548 the term Fe2O3 shell should be replaced by Fe2O3 core