Abstract
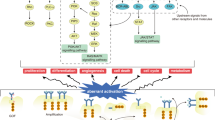
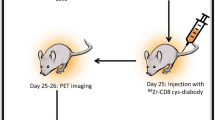
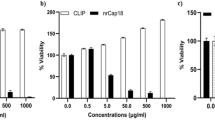
Positron emission tomography (PET) is a sensitive and noninvasive imaging method that is widely used to explore molecular events in living subjects. PET can precisely and quantitatively evaluate cellular apoptosis, which has a crucial role in various physiological and pathological processes. In this protocol, we describe the design and use of an engineered cyclic herpes simplex virus 1–thymidine kinase (HSV1-TK) PET reporter whose kinase activity is specifically switched on by apoptosis. The expression of cyclic TK (cTK) in healthy cells leads to inactive product, whereas the activation of apoptosis through the caspase-3 pathway cleaves cTK, thus restoring its activity and enabling PET imaging. In addition to detailing the design and construction of the cTK plasmid in this protocol, we include assays for evaluating the function and specificity of the cTK reporter in apoptotic cells, such as assays for measuring the cell uptake of PET tracer in apoptotic cells, correlating doxorubicin (Dox)-induced cell apoptosis to cTK function recovery, and in vivo PET imaging of cancer cell apoptosis, and we also include corresponding data acquisition methods. The time to build the entire cTK reporter is ∼2–3 weeks. The selection of a stable cancer cell line takes ∼4–6 weeks. The time to implement assays regarding cTK function in apoptotic cells and the in vivo imaging varies depending on the experiment. The cyclization strategy described in this protocol can also be adapted to create other reporter systems for broad biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Niu, G. & Chen, X. Molecular imaging with activatable reporter systems. Theranostics 2, 413–423 (2012).
Massoud, T.F. & Gambhir, S.S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580 (2003).
Jones, T. The imaging science of positron emission tomography. Eur. J. Nucl. Med. 23, 807–813 (1996).
Danial, N.N. & Korsmeyer, S.J. Cell death: critical control points. Cell 116, 205–219 (2004).
Dive, C., Evans, C.A. & Whetton, A.D. Induction of apoptosis—new targets for cancer chemotherapy. Semin. Cancer Biol. 3, 417–427 (1992).
Evan, G.I. & Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Green, A.M. & Steinmetz, N.D. Monitoring apoptosis in real time. Cancer J. 8, 82–92 (2002).
Blankenberg, F.G. & Strauss, H.W. Recent advances in the molecular imaging of programmed cell death: part I–pathophysiology and radiotracers. J. Nucl. Med. 53, 1659–1662 (2012).
Blankenberg, F.G. & Strauss, H.W. Recent advances in the molecular imaging of programmed cell death: part II–non-probe-based MRI, ultrasound, and optical clinical imaging techniques. J. Nucl. Med. 54, 1–4 (2013).
Blankenberg, F.G. Imaging the molecular signatures of apoptosis and injury with radiolabeled annexin V. Proc. Am. Thorac. Soc. 6, 469–476 (2009).
Hu, S. et al. Longitudinal PET imaging of doxorubicin-induced cell death with 18F-annexin V. Mol. Imaging Biol. 14, 762–770 (2012).
Toretsky, J. et al. Preparation of F-18 labeled annexin V: a potential PET radiopharmaceutical for imaging cell death. Nucl. Med. Biol. 31, 747–752 (2004).
Hoglund, J. et al. 18F-ML-10, a PET tracer for apoptosis: first human study. J. Nucl. Med. 52, 720–725 (2011).
Krysko, O., De Ridder, L. & Cornelissen, M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis 9, 495–500 (2004).
Riedl, S.J. & Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5, 897–907 (2004).
Su, H. et al. Evaluation of [18F]-CP18 as a PET imaging tracer for apoptosis. Mol. Imaging Biol. 15, 739–747 (2013).
Edgington, L.E. et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 15, 967–973 (2009).
Laxman, B. et al. Noninvasive real-time imaging of apoptosis. Proc. Natl. Acad. Sci. USA 99, 16551–16555 (2002).
Shen, B. et al. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-triggered nanoaggregation probe. Angew. Chem. Int. Ed. Engl. 52, 10511–10514 (2013).
Xia, C.F. et al. In vitro and in vivo evaluation of the caspase-3 substrate-based radiotracer [18F]-CP18 for PET imaging of apoptosis in tumors. Mol. Imaging Biol. 15, 748–757 (2013).
Challapalli, A. et al. 18F-ICMT-11, a caspase-3-specific PET tracer for apoptosis: biodistribution and radiation dosimetry. J. Nucl. Med. 54, 1551–1556 (2013).
Nguyen, Q.D., Challapalli, A., Smith, G., Fortt, R. & Aboagye, E.O. Imaging apoptosis with positron emission tomography: ′bench to bedside′ development of the caspase-3/7 specific radiotracer [18F]ICMT-11. Eur. J. Cancer 48, 432–440 (2012).
Bullok, K.E. et al. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry 46, 4055–4065 (2007).
Barnett, E.M., Zhang, X., Maxwell, D., Chang, Q. & Piwnica-Worms, D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc. Natl. Acad. Sci. USA 106, 9391–9396 (2009).
Bardet, P.L. et al. A fluorescent reporter of caspase activity for live imaging. Proc. Natl. Acad. Sci. USA 105, 13901–13905 (2008).
Niu, G. et al. Longitudinal bioluminescence imaging of the dynamics of doxorubicin-induced apoptosis. Theranostics 3, 190–200 (2013).
Yaghoubi, S.S. & Gambhir, S.S. Measuring herpes simplex virus thymidine kinase reporter gene expression in vitro. Nat. Protoc. 1, 2137–2142 (2006).
Zhang, X.X. et al. Comparison of 18F-labeled CXCR4 antagonist peptides for PET imaging of CXCR4 expression. Mol. Imaging Biol. 15, 758–767 (2013).
Xiong, Z. et al. Imaging chemically modified adenovirus for targeting tumors expressing integrin αvβ3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J. Nucl. Med. 47, 130–139 (2006).
Tjuvajev, J.G. et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 56, 4087–4095 (1996).
Cao, F. et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 113, 1005–1014 (2006).
Kang, K.W., Min, J.J., Chen, X. & Gambhir, S.S. Comparison of [14C]FMAU, [3H]FEAU, [14C]FIAU, and [3H]PCV for monitoring reporter gene expression of wild type and mutant herpes simplex virus type 1 thymidine kinase in cell culture. Mol. Imaging Biol. 7, 296–303 (2005).
Chin, F.T. et al. Semiautomated radiosynthesis and biological evaluation of [18F]FEa novel PET imaging agent for HSV1-tk/sr39tk reporter gene expression. Mol. Imaging Biol. 10, 82–91 (2008).
Wang, F. et al. A cyclic HSV1-TK reporter for real-time PET imaging of apoptosis. Proc. Natl. Acad. Sci. USA 111, 5165–5170 (2014).
Eroshenko, N. & Church, G.M. Mutants of Cre recombinase with improved accuracy. Nat. Commun. 4, 2509 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Yang, H., Wang, H. & Jaenisch, R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 (2014).
Ittner, L.M. & Gotz, J. Pronuclear injection for the production of transgenic mice. Nat. Protoc. 2, 1206–1215 (2007).
Li, L. & Blankenstein, T. Generation of transgenic mice with megabase-sized human yeast artificial chromosomes by yeast spheroplast-embryonic stem cell fusion. Nat. Protoc. 8, 1567–1582 (2013).
Wu, H., Hu, Z. & Liu, X.Q. Protein trans-splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 95, 9226–9231 (1998).
Cowsill, C. et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Ther. 7, 679–685 (2000).
Rogers, S., Wells, R. & Rechsteiner, M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234, 364–368 (1986).
Li, X. et al. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 273, 34970–34975 (1998).
Wild, K., Bohner, T., Folkers, G. & Schulz, G.E. The structures of thymidine kinase from herpes simplex virus type 1 in complex with substrates and a substrate analogue. Protein Sci. 6, 2097–2106 (1997).
Wurth, C., Thomas, R.M., Folkers, G. & Scapozza, L. Folding and self-assembly of herpes simplex virus type 1 thymidine kinase. J. Mol. Biol. 313, 657–670 (2001).
Wang, Z. et al. Biomimetic RNA-silencing nanocomplexes: overcoming multidrug resistance in cancer cells. Angew Chem. Int. Ed. Engl. 53, 1997–2001 (2014).
Nicholson, D.W. et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 (1995).
Wang, Z., Chui, W.K. & Ho, P.C. Design of a multifunctional PLGA nanoparticulate drug delivery system: evaluation of its physicochemical properties and anticancer activity to malignant cancer cells. Pharm. Res. 26, 1162–1171 (2009).
Wang, Z. & Ho, P.C. Self-assembled core-shell vascular-targeted nanocapsules for temporal antivasculature and anticancer activities. Small 6, 2576–2583 (2010).
Liang, Y., Yan, C. & Schor, N.F. Apoptosis in the absence of caspase 3. Oncogene 20, 6570–6578 (2001).
Acknowledgements
This work was supported, in part, by the Center for Neuroscience and Regeneration Medicine Program at the Henry M. Jackson Foundation, and by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering, US National Institutes of Health (NIH). We thank T. Ozawa (University of Tokyo) for providing us with the cDNAs of DnaE and PEST.
Author information
Authors and Affiliations
Contributions
Z.W., N.H., G.N. and X.C. conceived and designed this research; Z.W., F.W., N.H. and G.N. performed the experiment; D.O.K. contributed new reagents and analytical tools; Z.W., F.W., G.N., J.T. and X.C. analyzed the data; and Z.W., G.N. and X.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Real-time PCR analysis cTK variant expression level in different stable selected cell colonies.
The number under each bar indicates the cTK variant at specific split sites, and the number in the bracket identifies the cell colony (n=3).
Supplementary Figure 2 Illustration of the stable cell selection procedure.
(Not drawn to scale.)
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 238 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, F., Hida, N. et al. Design of a functional cyclic HSV1-TK reporter and its application to PET imaging of apoptosis. Nat Protoc 10, 807–821 (2015). https://doi.org/10.1038/nprot.2015.048
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2015.048