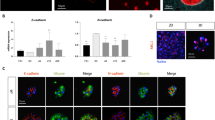
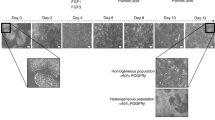
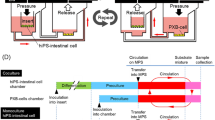
Abstract
The development of therapies and vaccines for human hepatropic pathogens requires robust model systems that enable the study of host-pathogen interactions. However, in vitro liver models of infection typically use either hepatoma cell lines that exhibit aberrant physiology or primary human hepatocytes in culture conditions in which they rapidly lose their hepatic phenotype. To achieve stable and robust in vitro primary human hepatocyte models, we developed micropatterned cocultures (MPCCs), which consist of primary human hepatocytes organized into 2D islands that are surrounded by supportive fibroblast cells. By using this system, which can be established over a period of days, and maintained over multiple weeks, we demonstrate how to recapitulate in vitro hepatic life cycles for the hepatitis B and C viruses and the Plasmodium pathogens P. falciparum and P. vivax. The MPCC platform can be used to uncover aspects of host-pathogen interactions, and it has the potential to be used for drug and vaccine development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lavanchy, D. The global burden of hepatitis C. Liver Int. 29 (suppl. 1), 74–81 (2009).
Ganem, D. & Prince, A.M. Hepatitis B virus infection—natural history and clinical consequences. N. Engl. J. Med. 350, 1118–1129 (2004).
Razavi, H. et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 57, 2164–2170 (2013).
Alonso, P.L. et al. A research agenda for malaria eradication: drugs. PLoS Med. 8, e1000402 (2011b).
Wells, T.N., Alonso, P.L. & Gutteridge, W.E. New medicines to improve control and contribute to the eradication of malaria. Nat. Rev. Drug Discov. 8, 879–891 (2009).
Wells, T.N., Burrows, J.N. & Baird, J.K. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 26, 145–151 (2010).
Billerbeck, E., de Jong, Y., Dorner, M., de la Fuente, C. & Ploss, A. Animal models for hepatitis C. Curr. Top. Microbiol. Immunol. 369, 49–86 (2013).
Kaushansky, A., Mikolajczak, S.A., Vignali, M. & Kappe, S.H. Of men in mice: the success and promise of humanized mouse models for human malaria parasite infections. Cell Microbiol. 16, 602–611 (2014).
Lohmann, V. & Bartenschlager, R. On the history of hepatitis C virus cell culture systems. J. Med. Chem. 57, 1627–1642 (2014).
Farkas, D. & Tannenbaum, S.R. In vitro methods to study chemically-induced hepatotoxicity: a literature review. Curr. Drug Metab. 6, 111–125 (2005).
Godoy, P. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87, 1315–1530 (2013).
Guillouzo, A. Liver cell models in in vitro toxicology. Environ. Health Perspect. 106 (suppl. 2), 511–532 (1998).
Khetani, S.R. et al. Microengineered liver tissues for drug testing. J. Lab. Autom. 20, 216–250 (2015).
Guillouzo, A. & Guguen-Guillouzo, C. Evolving concepts in liver tissue modeling and implications for in vitro toxicology. Expert Opin. Drug Metab. Toxicol. 4, 1279–1294 (2008).
LeCluyse, E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci. 13, 343–368 (2001).
Lagaye, S. et al. Efficient replication of primary or culture hepatitis C virus isolates in human liver slices: a relevant ex vivo model of liver infection. Hepatology 56, 861–872 (2012).
van Midwoud, P.M., Merema, M.T., Verweij, N., Groothuis, G.M. & Verpoorte, E. Hydrogel embedding of precision-cut liver slices in a microfluidic device improves drug metabolic activity. Biotechnol. Bioeng. 108, 1404–1412 (2011).
Gerets, H.H. et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 28, 69–87 (2012).
Wilkening, S., Stahl, F. & Bader, A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 31, 1035–1042 (2003).
Andersson, T.B., Kanebratt, K.P. & Kenna, J.G. The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin. Drug Metab. Toxicol. 8, 909–920 (2012).
Le Vee, M. et al. Functional expression of sinusoidal and canalicular hepatic drug transporters in the differentiated human hepatoma HepaRG cell line. Eur. J. Pharm. Sci. 28, 109–117 (2006).
Le Vee, M., Noel, G., Jouan, E., Stieger, B. & Fardel, O. Polarized expression of drug transporters in differentiated human hepatoma HepaRG cells. Toxicol. In Vitro 27, 1979–1986 (2013).
Lubberstedt, M. et al. HepaRG human hepatic cell line utility as a surrogate for primary human hepatocytes in drug metabolism assessment in vitro. J. Pharmacol. Toxicol. Methods 63, 59–68 (2011).
Szabo, M., Veres, Z., Baranyai, Z., Jakab, F. & Jemnitz, K. Comparison of human hepatoma HepaRG cells with human and rat hepatocytes in uptake transport assays in order to predict a risk of drug-induced hepatotoxicity. PLoS ONE 8, e59432 (2013).
Xu, J.J. et al. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 105, 97–105 (2008).
Bi, Y.A., Kazolias, D. & Duignan, D.B. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab. Dispos. 34, 1658–1665 (2006).
Dunn, J.C., Yarmush, M.L., Koebe, H.G. & Tompkins, R.G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 3, 174–177 (1989).
Khetani, S.R. & Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26, 120–126 (2008).
Sivaraman, A. et al. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr. Drug Metab. 6, 569–591 (2005).
LeCluyse, E.L., Witek, R.P., Andersen, M.E. & Powers, M.J. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 42, 501–548 (2012).
Ramsden, D., Zhou, J. & Tweedie, D.J. Determination of a degradation constant for CYP3A4 by direct suppression of mRNA in a novel human hepatocyte model, HepatoPac. Drug Metab. Dispos. 43, 1307–1315 (2015).
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 (2010).
Schwartz, R.E., Fleming, H.E., Khetani, S.R. & Bhatia, S.N. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol. Adv. 32, 504–513 (2014).
Cai, J. et al. in StemBook 1.52.1 (The Stem Cell Research Community) (Harvard Stem Cell Institute, 2012).
Berger, D.R., Ware, B.R., Davidson, M.D., Allsup, S.R. & Khetani, S.R. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61, 1370–1381 (2015).
Shan, J. et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat. Chem. Biol. 9, 514–520 (2013).
Bhatia, S.N. & Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Gripon, P. et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 99, 15655–15660 (2002).
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5, 215–229 (2005).
Gripon, P. et al. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J. Virol. 62, 4136–4143 (1988).
Banaudha, K. et al. Primary hepatocyte culture supports hepatitis C virus replication: a model for infection-associated hepatocarcinogenesis. Hepatology 51, 1922–1932 (2010).
Buck, M. Direct infection and replication of naturally occurring hepatitis C virus genotypes 1, 2, 3 and 4 in normal human hepatocyte cultures. PLoS ONE 3, e2660 (2008).
Harding, M.J. et al. An implantable vascularized protein gel construct that supports human fetal hepatoblast survival and infection by hepatitis C virus in mice. PLoS ONE 5, e9987 (2010).
Chattopadhyay, R. et al. Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS ONE 5, e14275 (2010).
Epstein, J.E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475 (2011).
Hollingdale, M.R., Collins, W.E. & Campbell, C.C. In vitro culture of exoerythrocytic parasites of the North Korean strain of Plasmodium vivax in hepatoma cells. Am. J. Trop. Med. Hyg. 35, 275–276 (1986).
Karnasuta, C. et al. Complete development of the liver stage of Plasmodium falciparum in a human hepatoma cell line. Am. J. Trop. Med. Hyg. 53, 607–611 (1995).
Sattabongkot, J. et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 74, 708–715 (2006).
Yokoo, H. et al. Proteomic signature corresponding to α-fetoprotein expression in liver cancer cells. Hepatology 40, 609–617 (2004).
Dembele, L. et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat. Med. 20, 307–312 (2014).
Mazier, D. et al. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 (1985).
Mazier, D. et al. Cultivation of the liver forms of Plasmodium vivax in human hepatocytes. Nature 307, 367–369 (1984).
van Schaijk, B.C. et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE 3, e3549 (2008).
Yalaoui, S. et al. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe 4, 283–292 (2008).
Bhatia, S.N., Balis, U.J., Yarmush, M.L. & Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 13, 1883–1900 (1999).
March, S. et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14, 104–115 (2013).
Ploss, A. et al. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc. Natl. Acad. Sci. USA 107, 3141–3145 (2010).
Shlomai, A. et al. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc. Natl. Acad. Sci. USA 111, 12193–12198 (2014).
Guguen-Guillouzo, C. et al. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp. Cell Res. 143, 47–54 (1983).
Rheinwald, J.G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343 (1975).
Khetani, S.R., Szulgit, G., Del Rio, J.A., Barlow, C. & Bhatia, S.N. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology 40, 545–554 (2004).
Leclercq, L. et al. Which human metabolites have we MIST? Retrospective analysis, practical aspects, and perspectives for metabolite identification and quantification in pharmaceutical development. Chem. Res. Toxicol. 22, 280–293 (2009).
Olson, H. et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 32, 56–67 (2000).
Shih, H., Pickwell, G.V., Guenette, D.K., Bilir, B. & Quattrochi, L.C. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum. Exp. Toxicol. 18, 95–105 (1999).
Wang, W.W., Khetani, S.R., Krzyzewski, S., Duignan, D.B. & Obach, R.S. Assessment of a micropatterned hepatocyte coculture system to generate major human excretory and circulating drug metabolites. Drug Metab. Dispos. 38, 1900–1905 (2010).
Khetani, S.R. et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol. Sci. 132, 107–117 (2013).
Chan, T.S., Yu, H., Moore, A., Khetani, S.R. & Tweedie, D. Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac. Drug Metab. Dispos. 41, 2024–2032 (2013).
Trask, O.J. Jr., Moore, A. & LeCluyse, E.L. A micropatterned hepatocyte coculture model for assessment of liver toxicity using high-content imaging analysis. Assay Drug Dev. Technol. 12, 16–27 (2014).
Ramsden, D., Tweedie, D.J., Chan, T.S., Taub, M.E. & Li, Y. Bridging in vitro and in vivo metabolism and transport of faldaprevir in human using a novel cocultured human hepatocyte system, HepatoPac. Drug Metab. Dispos. 42, 394–406 (2014).
Ng, S. et al. Hypoxia promotes liver-stage malaria infection in primary human hepatocytes in vitro. Dis. Model Mech. 7, 215–224 (2014).
Ukairo, O. et al. Long-term stability of primary rat hepatocytes in micropatterned cocultures. J. Biochem. Mol. Toxicol. 27, 204–212 (2013).
Aoyama, S., Lambirth, S. & Khetani, S.R. A long-term culture model for primary hepatoyctes from Cynomolgus hepatocytes. Drug Metab. Rev. 43, 124 (2011).
Dembele, L. et al. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS ONE 6, e18162 (2011).
Davidson, M.D., Lehrer, M. & Khetani, S.R. Hormone and drug-mediated modulation of glucose metabolism in a microscale model of the human liver. Tissue Eng. Part C Methods 21, 716–725 (2015).
Qin, D., Xia, Y. & Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
March, S., Hui, E.E., Underhill, G.H., Khetani, S. & Bhatia, S.N. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology 50, 920–928 (2009).
Chen, A.A., Khetani, S.R., Lee, S., Bhatia, S.N. & Van Vliet, K.J. Modulation of hepatocyte phenotype in vitro via chemomechanical tuning of polyelectrolyte multilayers. Biomaterials 30, 1113–1120 (2009).
Khetani, S.R., Chen, A.A., Ranscht, B. & Bhatia, S.N. T-cadherin modulates hepatocyte functions in vitro. FASEB J. 22, 3768–3775 (2008).
Meis, J.F. et al. Fine structure of the malaria parasite Plasmodium falciparum in human hepatocytes in vitro. Cell Tissue Res. 244, 345–350 (1986).
Shortt, H. History of recent researches on tissue phases of the malaria parasite at the London School of Hygiene and Tropical Medicine. Trans. R. Soc. Trop. Med. Hyg. 45, 175–188 (1951).
Shortt, H.E., Garnham, P.C. & Malamos, B. The pre-erythrocytic stage of mammalian malaria. Br. Med. J. 1, 192–194 (1948).
Vaughan, A.M. et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 122, 3618–3628 (2012).
Todaro, G.J. & Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313 (1963).
Bhatia, S.N., Yarmush, M.L. & Toner, M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res. 34, 189–199 (1997).
Zhong, J. et al. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102, 9294–9299 (2005).
Sells, M.A., Chen, M.L. & Acs, G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 84, 1005–1009 (1987).
Lindenbach, B.D. et al. Complete replication of hepatitis C virus in cell culture. Science 309, 623–626 (2005).
Jones, C.T. et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat. Biotechnol. 28, 167–171 (2010).
Albuquerque, S.S. et al. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics 10, 270 (2009).
Hoffman, S.L. et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccin. 6, 97–106 (2010).
Renia, L. et al. Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods 112, 201–205 (1988).
Silvie, O. et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat. Med. 9, 93–96 (2003).
Marukian, S. et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology 48, 1843–1850 (2008).
Glebe, D. et al. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J. Virol. 77, 9511–9521 (2003).
Portugal, S., Drakesmith, H. & Mota, M.M. Superinfection in malaria: Plasmodium shows its iron will. EMBO Rep. 12, 1233–1242 (2011).
Arzberger, S., Hosel, M. & Protzer, U. Apoptosis of hepatitis B virus-infected hepatocytes prevents release of infectious virus. J. Virol. 84, 11994–12001 (2010).
Reed, L.J. & Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497 (1938).
Acknowledgements
We thank R. Wirtz (Centers for Disease Control and Prevention) and F. Zavala (Johns Hopkins University) for PvCSP and HSP70 monoclonal antibodies, respectively, and the Malaria Research and Reference Reagent Resource Center (MR4) for monoclonal antibodies recognizing PfCSP (clone 2A10, deposited by E. Nardin) and EBA175 (clone R217, deposited by the US National Institute for Allergy and Infectious Disease (NIAID)). We are grateful to S. Hoffman and the Sanaria manufacturing team for the production of P. falciparum sporozoites and P. vivax sporozoites. We also thank J. Sattabongkot Prachumsri and R. Patrapuvich (Mahidol University) for providing fresh P. vivax sporozoites, and H. Green (Harvard University) for providing 3T3-J2 fibroblasts. This work has been supported in part by funding from the NIH (RO1 DK85713), a Skolkovo Institute of Science and Technology Grant 022423-003 and the Bill and Melinda Gates Foundation (OPP1023607). S.N.B is a Howard Hughes Medical Institute Investigator. S.R.K. acknowledges funding from the National Science Foundation (CAREER CBET-1351909) and Colorado State University. This paper is dedicated to the memory of Dr. Howard Green, a visionary and inspiring scientist.
Author information
Authors and Affiliations
Contributions
S.M., V.R., K.T., M.M.M., S.R.K., C.M.R. and S.N.B. conceived the study; S.M., V.R., K.T., S.N., M.S. and A.S. designed the experiments; malaria studies were conducted by S.M., S.N., N.G. and A.G.; hepatitis experiments were conducted by V.R., K.T., M.S. and A.S.; and the manuscript was prepared by S.M., V.R., K.T., N.G., S.R.K., H.E.F. and S.N.B.
Corresponding author
Ethics declarations
Competing interests
S.N.B. and S.R.K. are the founders of Hepregen Corporation. The remaining authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
March, S., Ramanan, V., Trehan, K. et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat Protoc 10, 2027–2053 (2015). https://doi.org/10.1038/nprot.2015.128
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2015.128
This article is cited by
-
Comparative analyses of functional antibody-mediated inhibition with anti-circumsporozoite monoclonal antibodies against transgenic Plasmodium berghei
Malaria Journal (2023)
-
Generation of proliferating human adult hepatocytes using optimized 3D culture conditions
Scientific Reports (2021)
-
Probing the distinct chemosensitivity of Plasmodium vivax liver stage parasites and demonstration of 8-aminoquinoline radical cure activity in vitro
Scientific Reports (2021)
-
Plasmodium vivax liver stage assay platforms using Indian clinical isolates
Malaria Journal (2020)
-
DEP-Dots for 3D cell culture: low-cost, high-repeatability, effective 3D cell culture in multiple gel systems
Scientific Reports (2020)