Abstract
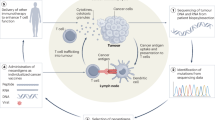
This protocol describes how to induce large numbers of tumor-specific cytotoxic T cells (CTLs) in the spleens and lymph nodes of mice receiving dendritic cell (DC) vaccines and how to modulate tumor microenvironments (TMEs) to ensure effective homing of the vaccination-induced CTLs to tumor tissues. We also describe how to evaluate the numbers of tumor-specific CTLs within tumors. The protocol contains detailed information describing how to generate a specialized DC vaccine with augmented ability to induce tumor-specific CTLs. We also describe methods to modulate the production of chemokines in the TME and show how to quantify tumor-specific CTLs in the lymphoid organs and tumor tissues of mice receiving different treatments. The combined experimental procedure, including tumor implantation, DC vaccine generation, chemokine-modulating (CKM) approaches, and the analyses of tumor-specific systemic and intratumoral immunity is performed over 30–40 d. The presented ELISpot-based ex vivo CTL assay takes 6 h to set up and 5 h to develop. In contrast to other methods of evaluating tumor-specific immunity in tumor tissues, our approach allows detection of intratumoral T-cell responses to nonmanipulated weakly immunogenic cancers. This detection method can be performed using basic laboratory skills, and facilitates the development and preclinical evaluation of new immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Johnson, L.A. & June, C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 27, 38–58 (2017).
Kalos, M. & June, C.H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 39, 49–60 (2013).
Maus, M.V. et al. Adoptive immunotherapy for cancer or viruses. Annu. Rev. Immunol. 32, 189–225 (2014).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Topalian, S.L. et al. Immunotherapy: the path to win the war on cancer? Cell 161, 185–186 (2015).
Tumeh, P.C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Herbst, R.S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Brahmer, J.R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Taube, J.M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Ji, R.R. et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 61, 1019–1031 (2012).
Gajewski, T.F., Schreiber, H. & Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Mailliard, R.B. et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 64, 5934–5937 (2004).
Giermasz, A.S. et al. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol. Immunother. 58, 1329–1336 (2009).
Lee, J.J., Foon, K.A., Mailliard, R.B., Muthuswamy, R. & Kalinski, P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J. Leukoc. Biol. 84, 319–325 (2008).
Muthuswamy, R. et al. NF-kappaB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 72, 3735–3743 (2012).
Muthuswamy, R., Corman, J.M., Dahl, K., Chatta, G.S. & Kalinski, P. Functional reprogramming of human prostate cancer to promote local attraction of effector CD8(+) T cells. Prostate 76, 1095–1105 (2016).
Muthuswamy, R., Wang, L., Pitteroff, J., Gingrich, J.R. & Kalinski, P. Combination of IFNα and poly-I:C reprograms bladder cancer microenvironment for enhanced CTL attraction. J. Immunother. Cancer 3, 6 (2015).
Downs-Canner, S. et al. Complement inhibition: a novel form of immunotherapy for colon cancer. Ann. Surg. Oncol. 23, 655–662 (2016).
Bach, S. et al. A yeast-based assay to isolate drugs active against mammalian prions. Methods 39, 72–77 (2006).
Galon, J., Fridman, W.H. & Pages, F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 67, 1883–1886 (2007).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Sato, E. et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 102, 18538–18543 (2005).
Fridman, W.H. et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 71, 5601–5605 (2011).
Fridman, W.H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Llosa, N.J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Le, D.T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Astsaturov, I. et al. Amplification of virus-induced antimelanoma T-cell reactivity by high-dose interferon-alpha2b: implications for cancer vaccines. Clin. Cancer Res. 9, 4347–4355 (2003).
Rosenberg, S.A. et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 175, 6169–6176 (2005).
Fox, B.A. et al. Defining the critical hurdles in cancer immunotherapy. J. Transl. Med. 9, 214 (2012).
Apetoh, L. et al. Consensus nomenclature for CD8+ T cell phenotypes in cancer. Oncoimmunology 4, e998538 (2015).
Kalinski, P., Muthuswamy, R. & Urban, J. Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev. Vaccines 12, 285–295 (2013).
Gajewski, T.F. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin. Oncol. 42, 663–671 (2015).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Fuertes, M.B., Woo, S.R., Burnett, B., Fu, Y.X. & Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 34, 67–73 (2013).
Woo, S.R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Ohkuri, T. et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol. Res. 2, 1199–1208 (2014).
Kreutz, M., Tacken, P.J. & Figdor, C.G. Targeting dendritic cells—why bother? Blood 121, 2836–2844 (2013).
Yang, X. et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell 25, 37–48 (2014).
Wong, J.L., Muthuswamy, R., Bartlett, D.L. & Kalinski, P. IL-18-based combinatorial adjuvants promote the intranodal production of CCL19 by NK cells and dendritic cells of cancer patients. Oncoimmunology 2, e26245 (2013).
Wong, J.L., Berk, E., Edwards, R.P. & Kalinski, P. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res. 73, 4653–4662 (2013).
Kalinski, P. & Gingrich, J.R. Toward improved effectiveness of bladder cancer immunotherapy. Immunotherapy 7, 1039–1042 (2015).
Lu, H. TLR agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front. Immunol. 5, 83 (2014).
Liu, Z. et al. CXCL11-armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology 5, e1091554 (2016).
Francis, L. et al. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget 7, 22174–22185 (2016).
Kantoff, P.W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Fong, L. et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl. Cancer Inst. 106 http://dx.doi.org/10.1093/jnci/dju268 (2014).
Inaba, K., Young, J.W. & Steinman, R.M. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J. Exp. Med. 166, 182–194 (1987).
Caux, C., Dezutter-Dambuyant, C., Schmitt, D. & Banchereau, J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360, 258–261 (1992).
Inaba, K. et al. Identification of proliferating dendritic cell precursors in mouse blood. J. Exp. Med. 175, 1157–1167 (1992).
Bender, A., Sapp, M., Schuler, G., Steinman, R.M. & Bhardwaj, N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods 196, 121–135 (1996).
Schuler, G. & Steinman, R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 186, 1183–1187 (1997).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179, 1109–1118 (1994).
Peters, J.H. et al. Signals required for differentiating dendritic cells from human monocytes in vitro. Adv. Exp. Med. Biol. 329, 275–280 (1993).
Brasel, K., De Smedt, T., Smith, J.L. & Maliszewski, C.R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96, 3029–3039 (2000).
Saunders, D. et al. Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 184, 2185–2196 (1996).
Maraskovsky, E. et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953–1962 (1996).
Brasel, K. et al. Hematologic effects of flt3 ligand in vivo in mice. Blood 88, 2004–2012 (1996).
Albert, M.L., Jegathesan, M. & Darnell, R.B. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2, 1010–1017 (2001).
Sparwasser, T. et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28, 2045–2054 (1998).
Binder, R.J., Anderson, K.M., Basu, S. & Srivastava, P.K. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J. Immunol. 165, 6029–6035 (2000).
Kalinski, P. et al. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Exp. Opin. Biol. Ther. 5, 1303–1315 (2005).
Jonuleit, H. et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27, 3135–3142 (1997).
Anguille, S., Smits, E.L., Lion, E., van Tendeloo, V.F. & Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 15, e257–e267 (2014).
Vieira, P.L., de Jong, E.C., Wierenga, E.A., Kapsenberg, M.L. & Kalinski, P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164, 4507–4512 (2000).
Kalinski, P., Schuitemaker, J.H., Hilkens, C.M. & Kapsenberg, M.L. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161, 2804–2809 (1998).
Kalinski, P., Vieira, P.L., Schuitemaker, J.H., de Jong, E.C. & Kapsenberg, M.L. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97, 3466–3469 (2001).
Whittaker, D.S., Bahjat, K.S., Moldawer, L.L. & Clare-Salzler, M.J. Autoregulation of human monocyte-derived dendritic cell maturation and IL-12 production by cyclooxygenase-2-mediated prostanoid production. J. Immunol. 165, 4298–4304 (2000).
Hilkens, C.M., Kalinski, P., de Boer, M. & Kapsenberg, M.L. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90, 1920–1926 (1997).
Kalinski, P., Hilkens, C.M., Snijders, A., Snijdewint, F.G. & Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159, 28–35 (1997).
Butterfield, L.H., Gooding, W. & Whiteside, T.L. Development of a potency assay for human dendritic cells: IL-12p70 production. J. Immunother. 31, 89–100 (2008).
Gustafsson, K. et al. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 68, 5965–5971 (2008).
Wesa, A., Kalinski, P., Kirkwood, J.M., Tatsumi, T. & Storkus, W.J. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J. Immunother. 30, 75–82 (2007).
Carreno, B.M. et al. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J. Clin. Invest. 123, 3383–3394 (2013).
Okada, H. et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 29, 330–336 (2011).
Zitvogel, L. et al. IL-12-engineered dendritic cells serve as effective tumor vaccine adjuvants in vivo. Ann. N. Y. Acad. Sci. 795, 284–293 (1996).
Xu, S. et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J. Immunol. 171, 2251–2261 (2003).
Ten Brinke, A., Karsten, M.L., Dieker, M.C., Zwaginga, J.J. & van Ham, S.M. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine 25, 7145–7152 (2007).
Chiang, C.L. et al. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J. Transl. Med. 9, 198 (2011).
Hokey, D.A., Larregina, A.T., Erdos, G., Watkins, S.C. & Falo, L.D. Jr. Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 65, 10059–10067 (2005).
Budiu, R.A. et al. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene 32, 3664–3675 (2013).
Schreibelt, G. et al. Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocyte-derived dendritic cells. Blood 116, 564–574 (2010).
Zitvogel, L. et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J. Exp. Med. 183, 87–97 (1996).
Mayordomo, J.I. et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1, 1297–1302 (1995).
Paglia, P., Chiodoni, C., Rodolfo, M. & Colombo, M.P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J. Exp. Med. 183, 317–322 (1996).
Fields, R.C., Shimizu, K. & Mule, J.J. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc. Natl. Acad. Sci. USA 95, 9482–9487 (1998).
Schnurr, M. et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res. 62, 2347–2352 (2002).
Strome, S.E. et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 62, 1884–1889 (2002).
Chiang, C.L., Coukos, G. & Kandalaft, L.E. Whole tumor antigen vaccines: where are we? Vaccines 3, 344–372 (2015).
Wieckowski, E. et al. Type-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8(+) T cells against prostate cancer cells and defined prostate cancer-specific epitopes. Prostate 71, 125–133 (2010).
Specht, J.M. et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J. Exp. Med. 186, 1213–1221 (1997).
Mullins, D.W. et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198, 1023–1034 (2003).
Okada, N. et al. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br. J. Cancer 84, 1564–1570 (2001).
Engleman, E.G. & Fong, L. Induction of immunity to tumor-associated antigens following dendritic cell vaccination of cancer patients. Clin. Immunol. 106, 10–15 (2003).
Fong, L., Brockstedt, D., Benike, C., Wu, L. & Engleman, E.G. Dendritic cells injected via different routes induce immunity in cancer patients. J. Immunol. 166, 4254–4259 (2001).
Bedrosian, I. et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J. Clin. Oncol. 21, 3826–3835 (2003).
Grover, A. et al. Intralymphatic dendritic cell vaccination induces tumor antigen-specific, skin-homing T lymphocytes. Clin. Cancer Res. 12, 5801–5808 (2006).
Radomski, M. et al. Prolonged intralymphatic delivery of dendritic cells through implantable lymphatic ports in patients with advanced cancer. J. Immunother. Cancer 4, 24 (2016).
Hu, J. et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 66, 8887–8896 (2006).
Nishioka, Y., Hirao, M., Robbins, P.D., Lotze, M.T. & Tahara, H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 59, 4035–4041 (1999).
Thompson, E.D., Enriquez, H.L., Fu, Y.X. & Engelhard, V.H. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J. Exp. Med. 207, 1791–1804 (2010).
Melero, I. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472 (2015).
Vasaturo, A., Verdoes, M., de Vries, J., Torensma, R. & Figdor, C.G. Restoring immunosurveillance by dendritic cell vaccines and manipulation of the tumor microenvironment. Immunobiology 220, 243–248 (2015).
Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Ribas, A. et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 15, 6267–6276 (2009).
Rosenblatt, J. et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 34, 409–418 (2011).
Wilgenhof, S. et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 34, 1330–1338 (2016).
Melero, I., Hervas-Stubbs, S., Glennie, M., Pardoll, D.M. & Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 7, 95–106 (2007).
Fujita, M. et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 71, 2664–2674 (2011).
Wong, J.L., Obermajer, N., Odunsi, K., Edwards, R.P. & Kalinski, P. Synergistic COX2 induction by IFNγ and TNFα self-limits type-1 immunity in the human tumor microenvironment. Cancer Immunol. Res. 4, 303–311 (2016).
Obermajer, N., Muthuswamy, R., Lesnock, J., Edwards, R.P. & Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118, 5498–5505 (2011).
Vacchelli, E. et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 3, e957994 (2014).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Li, J. et al. Chemokine expression from oncolytic Vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 19, 650–657 (2011).
Jensen, S.M. et al. Signaling through OX40 enhances antitumor immunity. Semin. Oncol. 37, 524–532 (2010).
Dranoff, G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nat. Rev. Immunol. 12, 61–66 (2011).
Ngiow, S.F., Loi, S., Thomas, D. & Smyth, M.J. Mouse models of tumor immunotherapy. Adv. Immunol. 130, 1–24 (2016).
Betts, M.R. et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281, 65–78 (2003).
Zaritskaya, L., Shurin, M.R., Sayers, T.J. & Malyguine, A.M. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev. Vaccines 9, 601–616 (2010).
Klenerman, P., Cerundolo, V. & Dunbar, P.R. Tracking T cells with tetramers: new tales from new tools. Nat. Rev. Immunol. 2, 263–272 (2002).
Karsunky, H., Merad, M., Cozzio, A., Weissman, I.L. & Manz, M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 (2003).
Pulendran, B. et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 96, 1036–1041 (1999).
Kalinski, P., Schuitemaker, J.H., Hilkens, C.M., Wierenga, E.A. & Kapsenberg, M.L. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J. Immunol. 162, 3231–3236 (1999).
Watchmaker, P.B. et al. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J. Immunol. 184, 591–597 (2010).
Hoffmann, T.K., Meidenbauer, N., Dworacki, G., Kanaya, H. & Whiteside, T.L. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 60, 3542–3549 (2000).
Albert, M.L. et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Snijders, A., Kalinski, P., Hilkens, C.M. & Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10, 1593–1598 (1998).
Wieckowski, E. et al. Type-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8(+) T cells against prostate cancer cells and defined prostate cancer-specific epitopes. Prostate 71, 125–133 (2011).
MartIn-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Wei, W.Z., Jones, R.F., Juhasz, C., Gibson, H. & Veenstra, J. Evolution of animal models in cancer vaccine development. Vaccine 33, 7401–7407 (2015).
Mac Keon, S., Ruiz, M.S., Gazzaniga, S. & Wainstok, R. Dendritic cell-based vaccination in cancer: therapeutic implications emerging from murine models. Front. Immunol. 6, 243 (2015).
Westwood, J.A., Darcy, P.K. & Kershaw, M.H. The potential impact of mouse model selection in preclinical evaluation of cancer immunotherapy. Oncoimmunology 3, e946361 (2014).
Devaud, C. et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol. Ther. 22, 18–27 (2014).
Devaud, C., John, L.B., Westwood, J.A., Darcy, P.K. & Kershaw, M.H. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2, e25961 (2013).
Rakoff-Nahoum, S. & Medzhitov, R. Toll-like receptors and cancer. Nat. Rev. Cancer 9, 57–63 (2009).
Lou, Y. et al. Antitumor activity mediated by CpG: the route of administration is critical. J. Immunother. 34, 279–288 (2011).
Amos, S.M. et al. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol. Immunother. 60, 671–683 (2011).
John, L.B. et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 72, 1651–1660 (2012).
Bartlett, D.L. et al. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer 12, 103 (2013).
Kirn, D.H. & Thorne, S.H. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer 9, 64–71 (2009).
Kerkar, S.P. et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 121, 4746–4757 (2011).
Zhang, L. et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol. Ther. 19, 751–759 (2011).
Lotze, M.T. et al. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann. N. Y. Acad. Sci. 795, 440–454 (1996).
Schlom, J. Therapeutic cancer vaccines: current status and moving forward. J. Natl. Cancer Inst. 104, 599–613 (2012).
Datta, J. et al. Rationale for a multimodality strategy to enhance the efficacy of dendritic cell-based cancer immunotherapy. Front. Immunol. 6, 271 (2015).
Mach, N. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Lane, P., Burdet, C., McConnell, F., Lanzavecchia, A. & Padovan, E. CD40 ligand-independent B cell activation revealed by CD40 ligand-deficient T cell clones: evidence for distinct activation requirements for antibody formation and B cell proliferation. Eur. J. Immunol. 25, 1788–1793 (1995).
Thirunavukarasu, P. et al. A rationally designed A34R mutant oncolytic poxvirus: improved efficacy in peritoneal carcinomatosis. Mol. Ther. 21, 1024–1033 (2013).
Wilhelm, K. et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 16, 1434–1438 (2010).
Kaka, A.S., Foster, A.E., Weiss, H.L., Rooney, C.M. & Leen, A.M. Using dendritic cell maturation and IL-12 producing capacity as markers of function: a cautionary tale. J. Immunother. 31, 359–369 (2008).
Nakamura, Y. et al. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 67, 10012–10018 (2007).
Musha, H. et al. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int. J. Cancer 116, 949–956 (2005).
Kunz, M. et al. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J. Pathol. 189, 552–558 (1999).
Ohtani, H., Jin, Z., Takegawa, S., Nakayama, T. & Yoshie, O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. J. Pathol. 217, 21–31 (2009).
Sampath, P. et al. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol. Ther. 21, 620–628 (2013).
Obermajer, N., Muthuswamy, R., Odunsi, K., Edwards, R.P. & Kalinski, P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 71, 7463–7470 (2011).
Acknowledgements
We dedicate this article to Eva Wieckowski (who died unexpectedly on 14 December 2015). We thank T.J. Curiel for providing the ID8A ovarian cancer cell line, P. Lane for CD40L-expressing J558 cells, and M. Kronenberg for Flt3-L-expressing B16 cells. We thank K. Lemon for assistance with monitoring the mice and BLI, and P. Bailey for critical review and editorial comments. This work was supported by NIH grant CA132714 (to P.K.), by a 2015 CRI Clinical Strategy Team Grant (to P.K.), and by the David C. Koch Regional Therapy Cancer Center (D.L.B.).
Author information
Authors and Affiliations
Contributions
N.O., J.U., and R.R. participated in data generation; N.O. and J.U. selected and evaluated the experimental data; N.O., J.U., R.M., R.R., E.W., P.K., and D.L.B. participated in the design of the protocol; N.O. and P.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Methods of production and clinical use of human variants of type-1 polarized DCs are a subject of two US patents (to P.K.). The remaining authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Obermajer, N., Urban, J., Wieckowski, E. et al. Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents. Nat Protoc 13, 335–357 (2018). https://doi.org/10.1038/nprot.2017.130
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/nprot.2017.130
This article is cited by
-
Predicting prostate adenocarcinoma patients’ survival and immune signature: a novel risk model based on telomere-related genes
Discover Oncology (2024)
-
Microbial synthesis of Prussian blue for potentiating checkpoint blockade immunotherapy
Nature Communications (2023)
-
A novel CTLA-4 blocking strategy based on nanobody enhances the activity of dendritic cell vaccine-stimulated antitumor cytotoxic T lymphocytes
Cell Death & Disease (2023)
-
Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors
npj Breast Cancer (2022)
-
The application of tumor cell-derived vesicles in oncology therapy
Clinical and Translational Oncology (2022)