Key Points
-
Zebrafish develop cancer that is histologically and genetically similar to that of humans and it can develop spontaneously, after mutagen exposure or through transgenesis.
-
Large-scale transgenesis using hundreds to thousands of embryos per day allows for deep functional characterization of genetic abnormalities that have been discovered in human cancer studies, such as The Cancer Genome Atlas.
-
Chemical screens have successfully been used in zebrafish embryos to find molecules and pathways that are directly relevant to human malignancy, and drugs from these screens have entered clinical trials in patients.
-
Large-scale forward genetic screens offer a unique opportunity to identify cancer phenotypes in an unbiased manner.
-
Transparent embryos and adults allows for in vivo visualization of cancer growth and progression at single-cell resolution.
-
Xenotransplantation of human cancer cells into zebrafish larvae provides opportunities for personalized cancer screens.
-
Major areas of growth in the coming decade include modelling multigenic changes in cancer, epigenetics and the dissection of metastasis.
Abstract
The zebrafish is a recent addition to animal models of human cancer, and studies using this model are rapidly contributing major insights. Zebrafish develop cancer spontaneously, after mutagen exposure and through transgenesis. The tumours resemble human cancers at the histological, gene expression and genomic levels. The ability to carry out in vivo imaging, chemical and genetic screens, and high-throughput transgenesis offers a unique opportunity to functionally characterize the cancer genome. Moreover, increasingly sophisticated modelling of combinations of genetic and epigenetic alterations will allow the zebrafish to complement what can be achieved in other models, such as mouse and human cell culture systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Ding, L. et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481, 506–510 (2012).
Berger, M. F. et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature 485, 502–506 (2012).
Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 (1996).
Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996). References 4 and 5 are landmark papers describing the use of the zebrafish in a phenotypic forward genetic screen.
Dimitrijevic, N. et al. Activation of the Xmrk proto-oncogene of Xiphophorus by overexpression and mutational alterations. Oncogene 16, 1681–1690 (1998).
Pliss, G. B., Zabezhinski, M. A., Petrov, A. S. & Khudoley, V. V. Peculiarities of N-nitramines carcinogenic action. Arch. Geschwulstforsch 52, 629–634 (1982).
Beckwith, L. G., Moore, J. L., Tsao-Wu, G. S., Harshbarger, J. C. & Cheng, K. C. Ethylnitrosourea induces neoplasia in zebrafish (Danio rerio). Lab Invest. 80, 379–385 (2000).
Spitsbergen, J. M. et al. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol. Pathol. 28, 705–715 (2000).
Spitsbergen, J. M. et al. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N′-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol. Pathol. 28, 716–725 (2000).
Culp, P., Nusslein-Volhard, C. & Hopkins, N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc. Natl Acad. Sci. USA 88, 7953–7957 (1991).
Lin, S., Yang, S. & Hopkins, N. lacZ expression in germline transgenic zebrafish can be detected in living embryos. Dev. Biol. 161, 77–83 (1994).
Lin, S. et al. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science 265, 666–669 (1994).
Langenau, D. M. et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 299, 887–890 (2003). The first description of a transgenic cancer model in the zebrafish.
Berghmans, S. et al. Making waves in cancer research: new models in the zebrafish. Biotechniques 39, 227–237 (2005).
Stoletov, K. & Klemke, R. Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 (2008).
Berghmans, S. et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl Acad. Sci. USA 102, 407–412 (2005). The first description of a conserved tumour-suppressor function of p53 in zebrafish.
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Lin, W. M. et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 68, 664–673 (2008).
Ceol, C. J. et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 471, 513–517 (2011). An in vivo genetic overexpression screen that used human oncogenomic data with in vivo melanoma modelling in the adult zebrafish.
Macgregor, S. et al. Genome-wide association study identifies a new melanoma susceptibility locus at 1q21.3. Nature Genet. 43, 1114–1118 (2011).
Liu, S. & Leach, S. D. Screening pancreatic oncogenes in zebrafish using the Gal4/UAS system. Methods Cell Biol. 105, 367–381 (2011).
Zhu, S. et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 21, 362–373 (2012).
Rudner, L. A. et al. Shared acquired genomic changes in zebrafish and human T-ALL. Oncogene 30, 4289–4296 (2011).
Lam, S. H. et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nature Biotech. 24, 73–75 (2006). A comprehensive analysis demonstrating the conservation of transcriptomic signatures between zebrafish and human liver cancer.
Lam, S. H. & Gong, Z. Modeling liver cancer using zebrafish: a comparative oncogenomics approach. Cell Cycle 5, 573–577 (2006).
Dovey, M., White, R. M. & Zon, L. I. Oncogenic NRAS cooperates with p53 loss to generate melanoma in zebrafish. Zebrafish 6, 397–404 (2009).
White, R. M. et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471, 518–522 (2011). A chemical genetic screen in zebrafish to identify small-molecule suppressors of melanoma progenitors, the results of which have led to human clinical trials.
Langenau, D. M. et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 21, 1382–1395 (2007).
Santoriello, C. et al. Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS ONE 5, e15170 (2010).
Patton, E. E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005).
Mizgireuv, I. V. & Revskoy, S. Y. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 66, 3120–3125 (2006).
Mizgirev, I. & Revskoy, S. Generation of clonal zebrafish lines and transplantable hepatic tumors. Nature Protoc. 5, 383–394 (2010).
Sabaawy, H. E. et al. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc. Natl Acad. Sci. USA 103, 15166–15171 (2006).
Gill, J. A. et al. Enforced expression of Simian virus 40 large T-antigen leads to testicular germ cell tumors in zebrafish. Zebrafish 7, 333–341 (2010).
Nguyen, A. T. et al. An inducible krasV12 transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis. Model. Mech. 5, 63–72 (2012).
Ignatius, M. S. et al. In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell 21, 680–693 (2012).
White, R. M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008). A paper describing the development of the transparent casper transplant model.
Feng, H. et al. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell 18, 353–366 (2010).
Smith, A. C. et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 115, 3296–3303 (2010).
Marques, I. J. et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 9, 128 (2009). A demonstration of the feasibility of transplanting human cancer cells into embryonic zebrafish as a readout for metastatic capacity.
Vlecken, D. H. & Bagowski, C. P. LIMK1 and LIMK2 are important for metastatic behavior and tumor cell-induced angiogenesis of pancreatic cancer cells. Zebrafish 6, 433–439 (2009).
Rouhi, P. et al. Pathological angiogenesis facilitates tumor cell dissemination and metastasis. Cell Cycle 9, 913–917 (2010).
Stoletov, K. et al. Visualizing extravasation dynamics of metastatic tumor cells. J. Cell Sci. 123, 2332–2341 (2010).
Zhao, C. et al. A novel xenograft model in zebrafish for high-resolution investigating dynamics of neovascularization in tumors. PLoS ONE 6, e21768 (2011).
Zhao, C. et al. Distinct contributions of angiogenesis and vascular co-option during the initiation of primary microtumors and micrometastases. Carcinogenesis 32, 1143–1150 (2011).
Eguiara, A. et al. Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 10, 3751–3757 (2011).
Ghotra, V. P. et al. Automated whole animal bio-imaging assay for human cancer dissemination. PLoS ONE 7, e31281 (2012).
Topczewska, J. M. et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nature Med. 12, 925–932 (2006).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nature Rev. Cancer 9, 239–252 (2009).
Choorapoikayil, S., Kuiper, R. V., de Bruin, A. & den Hertog, J. Haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes zebrafish to hemangiosarcoma. Dis. Model. Mech. 5, 241–247 (2012).
Patton, E. E. Live imaging in zebrafish reveals neu(trophil) insight into the metastatic niche. J. Pathol. 227, 381–384 (2012).
Bertrand, J. Y. et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147–4156 (2007).
Murphy, E. A. et al. Disruption of angiogenesis and tumor growth with an orally active drug that stabilizes the inactive state of PDGFRβ/B-RAF. Proc. Natl Acad. Sci. USA 107, 4299–4304 (2010).
Corkery, D. P., Dellaire, G. & Berman, J. N. Leukaemia xenotransplantation in zebrafish--chemotherapy response assay in vivo. Br. J. Haematol. 153, 786–789 (2011).
Wang, C. et al. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur. Urol. 58, 418–426 (2010).
Stoletov, K., Montel, V., Lester, R. D., Gonias, S. L. & Klemke, R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl Acad. Sci. USA 104, 17406–17411 (2007).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Adatto, I., Lawrence, C., Thompson, M. & Zon, L. I. A new system for the rapid collection of large numbers of developmentally staged zebrafish embryos. PLoS ONE 6, e21715 (2011).
Van Leeuwen, C. J., Grootelaar, E. M. & Niebeek, G. Fish embryos as teratogenicity screens: a comparison of embryotoxicity between fish and birds. Ecotoxicol. Environ. Saf 20, 42–52 (1990).
Peterson, R. T., Link, B. A., Dowling, J. E. & Schreiber, S. L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl Acad. Sci. USA 97, 12965–12969 (2000). The first systematic chemical screen in zebrafish.
Stern, H. M. et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nature Chem. Biol. 1, 366–370 (2005).
Saville, M. K. & Watson, R. J. B-Myb: a key regulator of the cell cycle. Adv. Cancer Res. 72, 109–140 (1998).
Golicki, D. et al. Leflunomide in monotherapy of rheumatoid arthritis: meta-analysis of randomized trials. Pol. Arch. Med. Wewn. 122, 22–32 (2012).
Hong, S. K., Tsang, M. & Dawid, I. B. The mych gene is required for neural crest survival during zebrafish development. PLoS ONE 3, e2029 (2008).
Ridges, S. et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 119, 5621–5631 (2012).
Parant, J. M., George, S. A., Holden, J. A. & Yost, H. J. Genetic modeling of Li-Fraumeni syndrome in zebrafish. Dis. Model. Mech. 3, 45–56 (2010).
Zhang, G. et al. Highly aneuploid zebrafish malignant peripheral nerve sheath tumors have genetic alterations similar to human cancers. Proc. Natl Acad. Sci. USA 107, 16940–16945 (2010).
Amsterdam, A. et al. Identification of 315 genes essential for early zebrafish development. Proc. Natl Acad. Sci. USA 101, 12792–12797 (2004).
Amsterdam, A. et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2, E139 (2004).
Lai, K. et al. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Dev. Dyn. 238, 76–85 (2009).
Guertin, D. A. & Sabatini, D. M. Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 (2007).
Amsterdam, A. et al. Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Mol. Cancer Res. 7, 841–850 (2009).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Moore, J. L., Rush, L. M., Breneman, C., Mohideen, M. A. & Cheng, K. C. Zebrafish genomic instability mutants and cancer susceptibility. Genetics 174, 585–600 (2006).
Neumann, J. C., Dovey, J. S., Chandler, G. L., Carbajal, L. & Amatruda, J. F. Identification of a heritable model of testicular germ cell tumor in the zebrafish. Zebrafish 6, 319–327 (2009).
Shepard, J. L. et al. A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc. Natl Acad. Sci. USA 102, 13194–13199 (2005).
Shepard, J. L. et al. A mutation in separase causes genome instability and increased susceptibility to epithelial cancer. Genes Dev. 21, 55–59 (2007).
Neumann, J. C. et al. Mutation in the type IB bone morphogenetic protein receptor Alk6b impairs germ-cell differentiation and causes germ-cell tumors in zebrafish. Proc. Natl Acad. Sci. USA 108, 13153–13158 (2011).
Frazer, J. K. et al. Heritable T-cell malignancy models established in a zebrafish phenotypic screen. Leukemia 23, 1825–1835 (2009).
Miller, A. C., Obholzer, N. D., Shah, A. N., Megason, S. G. & Moens, C. B. RNA-seq-based mapping and candidate identification of mutations from forward genetic screens. Genome Res. 23, 679–686 (2013).
Hill, J. T. et al. MMAPPR: mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 23, 687–697 (2013).
Bowen, M. E., Henke, K., Siegfried, K. R., Warman, M. L. & Harris, M. P. Efficient mapping and cloning of mutations in zebrafish by low-coverage whole-genome sequencing. Genetics 190, 1017–1024 (2012).
McGrail, M. et al. Somatic mutagenesis with a Sleeping Beauty transposon system leads to solid tumor formation in zebrafish. PLoS ONE 6, e18826 (2011).
Bedell, V. M. et al. In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118 (2012).
Dahlem, T. J. et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8, e1002861 (2012).
De Rienzo, G., Gutzman, J. H. & Sive, H. Efficient shRNA-mediated inhibition of gene expression in zebrafish. Zebrafish 9, 97–107 (2012).
Premsrirut, P. K. et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 145, 145–158 (2011).
Shih, A. H., Abdel-Wahab, O., Patel, J. P. & Levine, R. L. The role of mutations in epigenetic regulators in myeloid malignancies. Nature Rev. Cancer 12, 599–612 (2012).
Ganis, J. J. et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev. Biol. 366, 185–194 (2012).
Wu, S. F. et al. DNA methylation profiling in zebrafish. Methods Cell Biol. 104, 327–339 (2011).
Goll, M. G. & Halpern, M. E. DNA methylation in zebrafish. Prog. Mol. Biol. Transl. Sci. 101, 193–218 (2011).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Feng, Y., Santoriello, C., Mione, M., Hurlstone, A. & Martin, P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 8, e1000562 (2010).
Park, S. W. et al. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology 134, 2080–2090 (2008).
Langenau, D. M. et al. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc. Natl Acad. Sci. USA 102, 6068–6073 (2005).
Feng, H. et al. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br. J. Haematol. 138, 169–175 (2007).
Chen, J. et al. NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia 21, 462–471 (2007).
Blackburn, J. S. et al. Notch signaling expands a pre-malignant pool of T-cell acute lymphoblastic leukemia clones without affecting leukemia-propagating cell frequency. Leukemia 26, 2069–2078 (2012).
Le, X. et al. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc. Natl Acad. Sci. USA 104, 9410–9415 (2007).
Ju, B., Spitsbergen, J., Eden, C. J., Taylor, M. R. & Chen, W. Co-activation of hedgehog and AKT pathways promote tumorigenesis in zebrafish. Mol. Cancer 8, 40 (2009).
Zhuravleva, J. et al. MOZ/TIF2-induced acute myeloid leukaemia in transgenic fish. Br. J. Haematol. 143, 378–382 (2008).
Chu, C. Y. et al. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS ONE 7, e36474 (2012).
Leacock, S. W. et al. A zebrafish transgenic model of Ewing's sarcoma reveals conserved mediators of EWS-FLI1 tumorigenesis. Dis. Model. Mech. 5, 95–106 (2012).
Li, Z. et al. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J. Hepatol 56, 419–425 (2012).
Yang, H. W. et al. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res. 64, 7256–7262 (2004).
Forrester, A. M. et al. NUP98-HOXA9-transgenic zebrafish develop a myeloproliferative neoplasm and provide new insight into mechanisms of myeloid leukaemogenesis. Br. J. Haematol. 155, 167–181 (2011).
Liu, N. A. et al. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc. Natl Acad. Sci. USA 108, 8414–8419 (2011).
He, S. et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 227, 431–445 (2012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
L.Z. is a founder and stockholder of Fate Therapeutics, and a founder and stockholder of Scholar Rock Inc., and a scientific adviser for Stemgent. R.W. and K.R. declare no competing financial interests.
Glossary
- Forward genetic screens
-
An approach in which germline mutations are induced by mutagens such as ethylnitrosourea and offspring are scored for phenotype of interest. The causal mutation is then identified in mutants with interesting phenotypes.
- GISTIC
-
(Genomic identification of significant targets in cancer). An algorithm for the identification of copy number gains and losses in human cancer, using genome-wide data such as high-density single nucleotide polymorphism arrays.
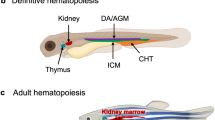
- Caudal haematopoietic tissue
-
(CHT). A region of the zebrafish embryo that acts as an intermediate site of embryonic blood development.
- TILLING
-
(Targeting induced local lesions in genomes). A technique to identify specific mutations in a gene of interest. Unbiased mutagenesis of the genome using ethylnitrosourea is carried out, followed by PCR-based sequencing of the region of interest to identify zebrafish with the preferred mutation.
- Li–Fraumeni syndrome
-
An autosomal-dominant condition caused by an inherited mutation in p53 that renders humans highly susceptible to a number of cancers, including breast cancer, leukaemia and sarcoma.
- CRISPR–Cas
-
Clustered regularly interspaced short palindromic repeat (CRISPR)–CRISPR-associated (Cas).
- miR-30-based approaches
-
A technique for improving the efficiency of in vivo RNA interference. A short hairpin RNA against a gene of interest is embedded within an endogenous microRNA-30 (miR-30) backbone, which allows for high-level expression from RNA polymerase II promoters.
Rights and permissions
About this article
Cite this article
White, R., Rose, K. & Zon, L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 13, 624–636 (2013). https://doi.org/10.1038/nrc3589
Published:
Issue date:
DOI: https://doi.org/10.1038/nrc3589
This article is cited by
-
Zebrafish xenograft as a tool for the study of colorectal cancer: a review
Cell Death & Disease (2024)
-
Myeloid differentiation factor-2/LY96, a potential predictive biomarker of metastasis and poor outcomes in prostate cancer: clinical implications as a potential therapeutic target
Oncogene (2024)
-
Design of an automated robotic microinjection system for batch injection of zebrafish embryos and larvae
Microsystems & Nanoengineering (2024)
-
Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets
Cancer and Metastasis Reviews (2023)
-
Identifying drivers of breast cancer metastasis in progressively invasive subpopulations of zebrafish-xenografted MDA-MB-231
Molecular Biomedicine (2022)