Key Points
-
Siglecs are sialic-acid-binding immunoglobulin-like lectins that are mostly expressed by cells of the immune system.
-
Siglecs can be divided into two subsets based on their sequence similarity and evolutionary relatedness. The main subset comprises the CD33-related subgroup, which appears to be evolving rapidly, and shows important differences in repertoire between mammalian species, particularly between humans and the closely related great apes.
-
Many Siglecs have tyrosine-based signalling motifs, especially immunoreceptor tyrosine-based inhibitory motifs (ITIMs), that are implicated in cell signalling and endocytosis.
-
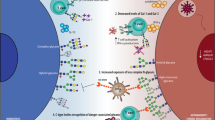
Low-affinity sialylated Siglec ligands are expressed abundantly on cells, which commonly results in the masking of the sialic-acid-binding site of Siglecs. Masking can be overcome by high-density and/or high-affinity ligands presented on opposing cells.
-
The Siglec CD22 is a well-characterized inhibitory receptor of B cells and has up to four ITIMs. Its cis interactions with α2–6-sialylated ligands are important for regulating signalling functions.
-
Sialoadhesin is a macrophage-restricted Siglec with 17 immunoglobulin domains. Its unusual length and the absence of signalling motifs suggest a predominant role in cell–cell interactions. This is also indicated in initial studies of sialoadhesin-deficient mice, which show impairment in development of experimentally induced autoimmune diseases.
-
The CD33-related Siglecs are expressed mostly in the innate immune system and appear to be important for regulating cellular expansion and activation. Most have two conserved tyrosine motifs, one of which is an ITIM important for recruitment of the SRC homology 2 (SH2)-domain-containing protein tyrosine phosphatase 1 (SHP1) and SHP2 tyrosine phosphatases and the suppressor of cytokine signalling 3 (SOCS3).
-
Many human pathogens can express sialic acids that are recognized by several Siglecs. It is possible that Siglec-dependent recognition of these pathogen glycans leads to altered immune responses, either to the advantage or to the detriment of the pathogen.
-
Some CD33-related Siglecs of the immune system, such as Siglec-H and Siglec-14, lack ITIMs. Instead, they associate with the immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor DAP12 that typically triggers cell activation. Siglec-14 is highly related to the ITIM-bearing Siglec-5 and they can therefore be considered as 'paired receptors'.
Abstract
Cell surfaces in the immune system are richly equipped with a complex mixture of glycans, which can be recognized by diverse glycan-binding proteins. The Siglecs are a family of sialic-acid-binding immunoglobulin-like lectins that are thought to promote cell–cell interactions and regulate the functions of cells in the innate and adaptive immune systems through glycan recognition. In this Review, we describe recent studies on signalling mechanisms and discuss the potential role of Siglecs in triggering endocytosis and in pathogen recognition. Finally, we discuss the postulated functions of the recently discovered CD33-related Siglecs and consider the factors that seem to be driving their rapid evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Williams, A. F. & Barclay, A. N. The immunoglobulin superfamily — domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405 (1988).
Powell, L. D. & Varki, A. I-type lectins. J. Biol. Chem. 270, 14243–14246 (1995).
Angata, T. & Brinkman-Van der Linden, E. I-type lectins. Biochim. Biophys. Acta 1572, 294–316 (2002).
Kelm, S. et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 (1994). This paper established the existence of the Siglec family, initially described as the sialoadhesin family.
Crocker, P. R. & Varki, A. Siglecs, sialic acids and innate immunity. Trends Immunol. 22, 337–342 (2001).
Crocker, P. R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell–cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002).
Crocker, P. R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5, 431–437 (2005).
Nitschke, L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr. Opin. Immunol. 17, 290–297 (2005).
Varki, A. & Angata, T. Siglecs — the major subfamily of I-type lectins. Glycobiology 16, 1R–27R (2006).
Angata, T., Margulies, E. H., Green, E. D. & Varki, A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl Acad. Sci. USA 101, 13251–13256 (2004). This extensive bioinformatics analysis on rodent and primate genome sequences establishes that the CD33-related Siglecs are rapidly evolving and exhibit striking differences in repertoire between mammalian species.
Angata, T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol. Divers. 10, 555–566 (2006).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Blasius, A. L., Cella, M., Maldonado, J., Takai, T. & Colonna, M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 107, 2474–2476 (2006).
Angata, T., Hayakawa, T., Yamanaka, M., Varki, A. & Nakamura, M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 20, 1964–1973 (2006).
Hamerman, J. A. & Lanier, L. L. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006, re1 (2006).
Bakker, T. R., Piperi, C., Davies, E. A. & Merwe, P. A. Comparison of CD22 binding to native CD45 and synthetic oligosaccharide. Eur. J. Immunol. 32, 1924–1932 (2002).
Blixt, O., Collins, B. E., van den Nieuwenhof, I. M., Crocker, P. R. & Paulson, J. C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278, 31007–31019 (2003).
Powell, L. D., Sgroi, D., Sjoberg, E. R., Stamenkovic, I. & Varki, A. Natural ligands of the B cell adhesion molecule CD22β carry N-linked oligosaccharides with α-2,6-linked sialic acids that are required for recognition. J. Biol. Chem. 268, 7019–7027 (1993). This paper shows that CD22, an IgSF member, is a sialic-acid-binding lectin with strict specificity for α2–6-sialylated glycans.
Kelm, S., Schauer, R., Manuguerra, J. C., Gross, H. J. & Crocker, P. R. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconj. J. 11, 576–585 (1994).
Yamaji, T., Teranishi, T., Alphey, M. S., Crocker, P. R. & Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to α2,8-disialyl and branched α2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 277, 6324–6332 (2002).
Hayakawa, T. et al. A human-specific gene in microglia. Science 309, 1693 (2005).
Bochner, B. S. et al. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 280, 4307–4312 (2005).
Tateno, H., Crocker, P. R. & Paulson, J. C. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 15, 1125–1135 (2005).
Campanero-Rhodes, M. A. et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem. Biophys. Res. Commun. 344, 1141–1146 (2006).
Rapoport, E. M., Pazynina, G. V., Sablina, M. A., Crocker, P. R. & Bovin, N. V. Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 71, 496–504 (2006).
Sonnenburg, J. L., Altheide, T. K. & Varki, A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology 14, 339–346 (2004).
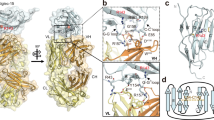
May, A. P., Robinson, R. C., Vinson, M., Crocker, P. R. & Jones, E. Y. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3' sialyllactose at 1.85 Å resolution. Mol. Cell 1, 719–728 (1998). In this paper, the first reported structure of a Siglec complexed with 3' sialyllactose ligand reveals how the immunoglobulin domain can adapt to sialic-acid binding.
Alphey, M. S., Attrill, H., Crocker, P. R. & van Aalten, D. M. High resolution crystal structures of Siglec-7. Insights into ligand specificity in the Siglec family. J. Biol. Chem. 278, 3372–3377 (2003).
Zaccai, N. R. et al. Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure 11, 557–567 (2003).
Attrill, H. et al. Siglec-7 undergoes a major conformational change when complexed with the α2,8-disialylganglioside GT1b. J. Biol. Chem. 281, 32774–32783 (2006).
Attrill, H. et al. The structure of siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design. Biochem. J. 397, 271–278 (2006).
Kelm, S., Gerlach, J., Brossmer, R., Danzer, C. P. & Nitschke, L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 195, 1207–1213 (2002).
Han, S., Collins, B. E., Bengtson, P. & Paulson, J. C. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nature Chem. Biol. 1, 93–97 (2005).
Collins, B. E. et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J. Immunol. 177, 2994–3003 (2006).
Collins, B. E., Smith, B. A., Bengtson, P. & Paulson, J. C. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nature Immunol. 7, 199–206 (2006). This paper, together with references 33, 55 and 85, examines the interplay between CD22, its sialylated ligands and the BCR in B-cell signalling.
Collins, B. E. et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl Acad. Sci. USA 101, 6104–6109 (2004).
Freeman, S. D., Kelm, S., Barber, E. K. & Crocker, P. R. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 85, 2005–2012 (1995). This is the first paper to show that CD33 can function as a sialic-acid binding lectin. Together with reference 38, this paper establishes that Siglecs can be masked when expressed at the cell surface and that unmasking can occur following sialidase-treatment of Siglec-expressing cells.
Hanasaki, K., Varki, A. & Powell, L. D. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by α2–6-sialylation of cellular glycoproteins. J. Biol. Chem. 270, 7533–7542 (1995).
Razi, N. & Varki, A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl Acad. Sci. USA 95, 7469–7474 (1998).
Razi, N. & Varki, A. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9, 1225–1234 (1999).
Munday, J., Floyd, H. & Crocker, P. R. Sialic acid binding receptors (siglecs) expressed by macrophages. J. Leukoc. Biol. 66, 705–711 (1999).
Lanoue, A., Batista, F. D., Stewart, M. & Neuberger, M. S. Interaction of CD22 with α2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur. J. Immunol. 32, 348–355 (2002).
Falco, M. et al. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 190, 793–802 (1999).
Nicoll, G. et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274, 34089–34095 (1999).
Avril, T., North, S. J., Haslam, S. M., Willison, H. J. & Crocker, P. R. Probing the cis interactions of the inhibitory receptor Siglec-7 with α2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of α2,8-sialyltransferase gene expression. J. Leukoc. Biol. 80, 787–796 (2006).
Nicoll, G. et al. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and-independent mechanisms. Eur. J. Immunol. 33, 1642–1648 (2003).
Crocker, P. R. & Feizi, T. Carbohydrate recognition systems: functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 6, 679–691 (1996).
Collins, B. E. et al. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology 12, 563–571 (2002).
Stamenkovic, I., Sgroi, D., Aruffo, A., Sy, M. S. & Anderson, T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2–6 sialyltransferase, CD75, on B cells. Cell 66, 1133–1144 (1991).
Hanasaki, K., Powell, L. D. & Varki, A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J. Biol. Chem. 270, 7543–7550 (1995).
Law, C. L., Aruffo, A., Chandran, K. A., Doty, R. T. & Clark, E. A. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J. Immunol. 155, 3368–3376 (1995).
Zhang, M. & Varki, A. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology 14, 939–949 (2004).
Powell, L. D., Jain, R. K., Matta, K. L., Sabesan, S. & Varki, A. Characterization of sialyloligosaccharide binding by recombinant soluble and native cell-associated CD22. Evidence for a minimal structural recognition motif and the potential importance of multisite binding. J. Biol. Chem. 270, 7523–7532 (1995).
John, B. et al. The B cell coreceptor CD22 associates with AP50, a clathrin-coated pit adapter protein, via tyrosine-dependent interaction. J. Immunol. 170, 3534–3543 (2003).
Grewal, P. K. et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol. Cell. Biol. 26, 4970–4981 (2006).
Vimr, E. & Lichtensteiger, C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10, 254–257 (2002).
Vimr, E. R., Kalivoda, K. A., Deszo, E. L. & Steenbergen, S. M. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68, 132–153 (2004).
Vanderheijden, N. et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 77, 8207–8215 (2003). This paper makes the surprising discovery that the sialylated porcine arterivirus depends on sialoadhesin for infection of macrophages.
Delputte, P. L. & Nauwynck, H. J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 78, 8094–8101 (2004).
Jones, C., Virji, M. & Crocker, P. R. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49, 1213–1225 (2003).
Monteiro, V. G. et al. Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitol. Res. 97, 380–385 (2005).
Avril, T., Wagner, E. R., Willison, H. J. & Crocker, P. R. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 74, 4133–4141 (2006).
Carlin, A. F., Lewis, A. L., Varki, A. & Nizet, V. Group B Streptococcal sialic acids interact with Siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 189, 1231–1237 (2007).
Crocker, P. R. & Gordon, S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 164, 1862–1875 (1986).
Crocker, P. R. et al. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 13, 4490–4503 (1994). This paper, together with reference 18, establishes that members of the IgSF can mediate sialic-acid-dependent adhesion.
Crocker, P. R. & Gordon, S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 169, 1333–1346 (1989).
Hartnell, A. et al. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97, 288–296 (2001).
Ikezumi, Y. et al. The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol. Dial. Transplant. 20, 2704–2713 (2005).
Ikezumi, Y. et al. Histological differences in newly onset IgA nephropathy between children and adults. Nephrol. Dial. Transplant. 20. 2704–2713 (2006).
Pulliam, L., Sun, B. & Rempel, H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 157, 93–98 (2004).
Jiang, H. R. et al. Sialoadhesin promotes the inflammatory response in experimental autoimmune uveoretinitis. J. Immunol. 177, 2258–2264 (2006).
Kobsar, I. et al. Attenuated demyelination in the absence of the macrophage-restricted adhesion molecule sialoadhesin (Siglec-1) in mice heterozygously deficient in P0. Mol. Cell. Neurosci. 31, 685–691 (2006).
Ip, C. W., Kroner, A., Crocker, P. R., Nave, K. A. & Martini, R. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol. Dis. 25, 105–111 (2007).
Nath, D. et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 98, 213–219 (1999).
van den Berg, T. K. et al. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1). J. Immunol. 166, 3637–3640 (2001).
Martinez-Pomares, L. et al. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J. Biol. Chem. 274, 35211–35218 (1999).
Kumamoto, Y. et al. Identification of sialoadhesin as a dominant lymph node counter-receptor for mouse macrophage galactose-type C-type lectin 1. J. Biol. Chem. 279, 49274–49280 (2004).
Kirchberger, S. et al. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J. Immunol. 175, 1145–1152 (2005).
Tedder, T. F., Poe, J. C. & Haas, K. M. CD22: A multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv. Immunol. 88, 1–50 (2005).
Doody, G. M. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995). This is one of the first papers to clearly demonstrate that CD22 functions as an inhibitory receptor of B cells via interactions with SHP1. It also shows that antibody-induced sequestration of CD22 away from the BCR lowers the threshold for B-cell activation by 100-fold.
Fujimoto, M. et al. B cell antigen receptor and CD40 differentially regulate CD22 tyrosine phosphorylation. J. Immunol. 176, 873–879 (2006).
Hokazono, Y. et al. Inhibitory coreceptors activated by antigens but not by anti-Ig heavy chain antibodies install requirement of costimulation through CD40 for survival and proliferation of B cells. J. Immunol. 171, 1835–1843 (2003).
Wakabayashi, C., Adachi, T., Wienands, J. & Tsubata, T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298, 2392–2395 (2002).
Poe, J. C. et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nature Immunol. 5, 1078–1087 (2004). This study examines the phenotype of knock-in mice bearing a mutated form of CD22 that is unable to bind sialic acids.
Ghosh, S., Bandulet, C. & Nitschke, L. Regulation of B cell development and B cell signalling by CD22 and its ligands α2,6-linked sialic acids. Int. Immunol. 18, 603–611 (2006).
Stoddart, A. et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462 (2002).
Stoddart, A., Jackson, A. P. & Brodsky, F. M. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol. Biol. Cell 16, 2339–2348 (2005).
Samardzic, T. et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 32, 561–567 (2002).
Erickson-Miller, C. L. et al. Characterization of Siglec-5 (CD170) expression and functional activity of anti-Siglec-5 antibodies on human phagocytes. Exp. Hematol. 31, 382–388 (2003).
Nguyen, D. H., Ball, E. D. & Varki, A. Myeloid precursors and acute myeloid leukemia cells express multiple CD33-related Siglecs. Exp. Hematol. 34, 728–735 (2006).
Biedermann, B., Gil, D., Bowen, D. T. & Crocker, P. R. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk. Res. 31, 211–220 (2007).
Vitale, C. et al. Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc. Natl Acad. Sci. USA 96, 15091–15096 (1999).
Balaian, L., Zhong, R. K. & Ball, E. D. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp. Hematol. 31, 363–371 (2003).
Nutku, E., Aizawa, H., Hudson, S. A. & Bochner, B. S. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101, 5014–5020 (2003).
von Gunten, S. et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106, 1423–1431 (2005). This paper and reference 94 are the first to show that ligation of Siglecs on granulocytes can trigger cell-death signals that are enhanced in the presence of cytokines that normally promote cell survival.
Paul, S. P., Taylor, L. S., Stansbury, E. K. & McVicar, D. W. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 96, 483–490 (2000).
Ulyanova, T., Shah, D. D. & Thomas, M. L. Molecular cloning of MIS, a myeloid inhibitory siglec, that binds protein-tyrosine phosphatases SHP-1 and SHP-2. J. Biol. Chem. 276, 14451–14458 (2001).
Avril, T., Floyd, H., Lopez, F., Vivier, E. & Crocker, P. R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and-9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173, 6841–6849 (2004).
Ikehara, Y., Ikehara, S. K. & Paulson, J. C. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 279, 43117–43125 (2004).
Avril, T., Freeman, S. D., Attrill, H., Clarke, R. G. & Crocker, P. R. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 280, 19843–19851 (2005).
Lajaunias, F., Dayer, J. M. & Chizzolini, C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 35, 243–251 (2005).
Orr, S. J. et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood 109, 1061–1068 (2007).
Orr, S. J. et al. SOCS3 targets Siglec 7 for proteasomal degradation and blocks Siglec 7-mediated responses. J. Biol. Chem. 282, 3418–3422 (2006).
von Gunten, S. & Simon, H. U. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 20, 601–605 (2006).
Nguyen, D. H., Hurtado-Ziola, N., Gagneux, P. & Varki, A. Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl Acad. Sci. USA 103, 7765–7770 (2006).
Zhang, J. Q., Biedermann, B., Nitschke, L. & Crocker, P. R. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur. J. Immunol. 34, 1175–1184 (2004).
Zhang, M. et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 1 Feb 2007 [epub ahead of print]. First demonstration of an inhibitory function for a CD33-related Siglec.
Lock, K., Zhang, J., Lu, J., Lee, S. H. & Crocker, P. R. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology 209, 199–207 (2004).
Zhang, J. et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 107, 3600–3608 (2006).
Walter, R. B., Raden, B. W., Kamikura, D. M., Cooper, J. A. & Bernstein, I. D. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 105, 1295–1302 (2005).
Patel, N. et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J. Biol. Chem. 274, 22729–22738 (1999).
Veillette, A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature Rev. Immunol. 6, 56–66 (2006).
Taylor, V. C. et al. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J. Biol. Chem. 274, 11505–11512 (1999). This paper establishes that a CD33-related Siglec can recruit the protein tyrosine phosphatases SHP1 and SHP2 to their ITIM and ITIM-like motif.
Yu, Z., Maoui, M., Wu, L., Banville, D. & Shen, S. mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. J. 353, 483–492 (2001).
Wong, P. K. et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J. Clin. Invest. 116, 1571–1581 (2006).
Yrlid, U. et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Crocker, P. R. et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology 8, v–vi (1998).
Blasius, A. L. & Colonna, M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 27, 255–260 (2006).
Tomasello, E. & Vivier, E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur. J. Immunol. 35, 1670–1677 (2005).
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002).
Smith, H. R. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl Acad. Sci. USA 99, 8826–8831 (2002).
Altheide, T. K. et al. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: Evidence for two modes of rapid evolution. J. Biol. Chem. 281, 25689–25702 (2006).
Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature (in the press).
Jones, G. et al. Polymorphisms within the CTLA4 gene are associated with infant atopic dermatitis. Br. J. Dermatol. 154, 467–471 (2006).
Kuroki, K. et al. Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA-DRB1 shared epitope negative rheumatoid arthritis. Hum. Mol. Genet. 14, 2469–2480 (2005).
Lin, S. C., Kuo, C. C. & Chan, C. H. Association of a BTLA gene polymorphism with the risk of rheumatoid arthritis. J. Biomed. Sci. 13, 853–860 (2006).
Tsuchiya, N., Honda, Z. & Tokunaga, K. Role of B cell inhibitory receptor polymorphisms in systemic lupus erythematosus: a negative times a negative makes a positive. J. Hum. Genet. 51, 741–750 (2006).
Crocker, P. R. et al. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 10, 1661–1669 (1991).
Acknowledgements
Work in the authors' laboratories is supported by grants from the Wellcome Trust (P.R.C.) and the National Institutes of Health, USA (J.P. and A.V.). We are grateful to T. Angata for communicating details of Siglec-15 ahead of publication and to H. Attrill for assistance in preparing Figures 2c and 2d.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- V-set immunoglobulin domain
-
A protein domain that shows evolutionary similarity, in both linear sequence and folded structure, to the variable region domains of immunoglobulins. The domain folds into a sandwich of two β-pleated sheets consisting of antiparallel β-strands. V-set domains differ from immunoglobulin constant-region type 2 (C2)-set domains by having more β-strands in the β-pleated sheets.
- C2-set immunoglobulin domain
-
A protein domain that shows evolutionary similarity, in both linear sequence and folded structure, to the immunoglobulin constant region type 2 (C2) domains. The domain folds into a sandwich of two β-pleated sheets consisting of anti-parallel β-strands. C2-set immunoglobulin domains differ from domains of the variable region of immunoglobulins by having fewer β-strands in the β-pleated sheets.
- Orthologue
-
Orthology describes genes in different species that derive from a common ancestor. Orthologous genes may or may not have the same function.
- The Red Queen effect
-
A term that describes unremitting evolutionary arms races that can occur between competing species, or between a pathogen and its host. The term is derived from the Red Queen's comment in Lewis Carroll's Through the Looking Glass: “It takes all the running you can do, to keep in the same place.”
- Immunoreceptor tyrosine-based inhibitory motif
-
(ITIM). A short peptide motif containing a tyrosine residue that is found in the cytoplasmic regions of many inhibitory receptors. The consensus sequence is (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val), with X denoting any amino acid. Following tyrosine phosphorylation by SRC-family protein tyrosine kinases, this provides a high-affinity docking site for the recruitment of cytoplasmic phosphatases and other signalling molecules with an appropriate SRC homology 2 (SH2) domain.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A short peptide motif containing tyrosine residues that is found in the cytoplasmic tails of several signalling molecules and in adaptors such as DAP12. The consensus sequence is (Asp/Glu)-X-X-Tyr-X-X-(Leu/Ile)-X6–8-Tyr-X-X-(Leu/Ile), with X denoting any amino acid. It is tyrosine phosphorylated after engagement of the ligand-binding subunits, which triggers a cascade of intracellular events that usually results in cellular activation.
- Glycome
-
The entire set of glycans in a cell, tissue or organism, under specified conditions. The sizes of glycomes are currently unknown but are likely to be many-fold larger than the size of the corresponding proteome owing to the combinatorial complexity and dynamic variability of glycan structures.
- Counter-receptor
-
A term used to describe the combination of oligosaccharide ligands coupled to protein or lipid carriers47. For many glycan-binding proteins, the affinity for oligosaccharide ligands is low, but high-avidity multivalent binding can occur when the ligands are appropriately displayed on carriers.
- Clathrin domains
-
Specialized membrane microdomains that mediate endocytosis to early endosomes by a mechanism involving the formation of clathrin cages on the cytoplasmic face of the plasma membrane.
- Proliferative glomerulonephritis
-
A group of inflammatory diseases affecting the glomerular apparatus of the kidney. These diseases have varied aetiologies but characteristically exhibit proliferation of mesangial cells and endocapillaries, and infiltration of leukocytes, such as macrophages and T cells.
- Experimental autoimmune uveoretinitis
-
A photoreceptor-specific autoimmune disease that is inducible in several susceptible animal models with various retinal autoantigens. It resembles some human posterior uveoretinitis syndromes, including sympathetic ophthalmia, Vogt–Koyanagi–Harada disease, sarcoidosis, Behçet's disease and birdshot retinochoroidopathy.
- Mixed lymphocyte reactions
-
A tissue-culture technique that is used for the in vitro testing of the proliferative response of T cells from one individual to lymphocytes from another individual.
- LYN-deficient mouse model of lupus
-
A deficiency of the phosphokinase LYN results in a hyperimmune status leading to an autoimmune condition that is similar to the human disease systemic lupus.
- Rafts or activation rafts
-
Membrane microdomains enriched in glycosphingolipids, where cell signalling receptors form macromolecular complexes with other proteins involved in the initiation of cell-activation pathways.
- Paralogue
-
Paralogy describes homologous genes in a single species that diverged by gene duplication. Paralogues are more likely to evolve new functions. Siglec-F and Siglec-8 are unusual in that they are paralogues that have developed similarities in cell-type expression and binding specificity by convergent evolution.
- Suppressors of cytokine signalling 3
-
(SOCS 3). A member of the family of eight cytoplasmic proteins (SOCS1–SOCS7 and CIS) that contain an amino-terminal region of variable length, a central SH2 domain and a carboxy-terminal SOCS box. SOCS proteins provide a negative-feedback loop to attenuate signal transduction from cytokine receptors that act through the JAK–STAT (Janus kinase –signal transducer and activator of transcription) pathway.
- Cross-presentation
-
The initiation of a CD8+ T-cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC-class-I pathway of antigen presentation.
- Paired receptors
-
These are membrane proteins, one of which is potentially inhibitory and the other activating and which are highly related to each other in the extracellular domain but differ significantly in the transmembrane and cytoplasmic regions.
Rights and permissions
About this article
Cite this article
Crocker, P., Paulson, J. & Varki, A. Siglecs and their roles in the immune system. Nat Rev Immunol 7, 255–266 (2007). https://doi.org/10.1038/nri2056
Issue date:
DOI: https://doi.org/10.1038/nri2056
This article is cited by
-
Identification of therapeutic targets and prognostic biomarkers in the Siglec family of genes in tumor immune microenvironment of sarcoma
Scientific Reports (2024)
-
The Contribution of Serum Sialic Acid Binding Immunoglobulin-Like Lectin 1(sSIGLEC-1) as an IFN I Signature Biomarker in the Progression of Atherosclerosis in Egyptian Systemic Lupus Erythematosus (SLE) Patients
Indian Journal of Clinical Biochemistry (2024)
-
CD33 as a leukocyte-associated marker expressed on human spermatozoa
BMC Research Notes (2023)
-
Polymeric biomaterial-inspired cell surface modulation for the development of novel anticancer therapeutics
Biomaterials Research (2023)
-
The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells
Nature (2023)