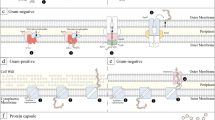
Abstract
Several medically important Gram-negative bacterial pathogens inject virulence factors into host cells through a type III secretion system and specialized bacterial chaperones are required for their effective delivery. Recent structural work shows that these chaperones maintain virulence factors in a partially non-globular conformation that is primed for unfolding and translocation through the 'injectisome'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cornelis, G. R. & Van Gijsegem, F. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54, 735–774 (2000).
Galán, J. E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328 (1999).
Kubori, T. et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605 (1998).
Rosqvist, R., Magnusson, K. E. & Wolf-Watz, H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13, 964–972 (1994).
Wattiau, P., Woestyn, S. & Cornelis, G. R. Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol. 20, 255–262 (1996).
Page, A. L. & Parsot, C. Chaperones of the type III secretion pathway: jacks of all trades. Mol. Microbiol. 46, 1–11 (2002).
Hartl, F. U. & Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002).
Menard, R., Sansonetti, P., Parsot, C. & Vasselon, T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 4, 515–525 (1994).
Tucker, S. C. & Galán, J. E. Complex function for SicA, a Salmonella enterica serovar typhimurium type III secretion-associated chaperone. J. Bacteriol. 182, 2262–2268 (2000).
Neyt, C. & Cornelis, G. R. Role of SycD, the chaperone of the Yersinia yop translocators YopB and YopD. Mol. Microbiol. 31, 143–156 (1999).
Bergman, T. et al. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173, 1607–1616 (1991).
Anderson, D. M., Ramamurthi, K. S., Tam, C. & Schneewind, O. YopD and LcrH regulate expression of Yersinia enterocolitica YopQ by a posttranscriptional mechanism and bind to yopQ RNA. J. Bacteriol. 184, 1287–1295 (2002).
Darwin, K. & Miller, V. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 20, 1850–1862 (2001).
Stebbins, C. E. & Galán, J. E. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 414, 77–81 (2001).
Luo, Y. et al. Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nature Struct. Biol. 8, 1031–1036 (2001).
Birtalan, S. & Ghosh, P. Structure of the Yersinia type III secretory system chaperone SycE. Nature Struct. Biol. 8, 974–978 (2001).
Evdokimov, A. G., Tropea, J. E., Routzahn, K. M. & Waugh, D. S. Three-dimensional structure of the type III secretion chaperone SycE from Yersinia pestis. Acta Crystallogr. D 58, 398–406 (2002).
Birtalan, S. C., Phillips, R. M. & Ghosh, P. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell. 9, 971–980 (2002).
Zierler, M. K. & Galán, J. E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect. Immun. 63, 4024–4028 (1995).
Bliska, J. B. & Black, D. S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63, 681–685 (1995).
Macbeth, K. J. & Lee, C. A. Prolonged inhibition of bacterial protein synthesis abolishes Salmonella invasion. Infect. Immun. 61, 1544–1546 (1993).
Blocker, A. et al. Structure and composition of the Shigella flexneri 'needle complex', a part of its type III secreton. Mol. Microbiol. 39, 652–663 (2001).
Cordes, F. S. et al. Helical structure of the needle of the type III secretion system of Shigella flexneri. J. Biol. Chem. 278, 17103–17107 (2003).
Fauman, E. B., Yuvaniyama, C., Schubert, H. L., Stuckey, J. A. & Saper, M. A. The X-ray crystal structures of Yersinia tyrosine phosphatase with bound tungstate and nitrate. Mechanistic implications. J. Biol. Chem. 271, 18780–18788 (1996).
Stebbins, C. E. & Galán, J. E. Modulation of host signaling by a bacterial mimic: structure of the Salmonella effector SptP bound to Rac1. Mol. Cell 6, 1449–1460 (2000).
Evdokimov, A. G., Anderson, D. E., Routzahn, K. M. & Waugh, D. S. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312, 807–821 (2001).
Samatey, F. A. et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 (2001).
Eichelberg, K., Ginocchio, C. & Galán, J. E. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J. Bacteriol. 176, 4501–4510 (1994).
Vogler, A. P., Homma, M., Irikura, V. M. & Macnab, R. M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of Flil to F0F1, vacuolar, and archaebacterial ATPase subunits. J. Bacteriol. 173, 3564–3572 (1991).
Wurtele, M., Renault, L., Barbieri, J. T., Wittinghofer, A. & Wolf, E. Structure of the ExoS GTPase activating domain. 491, 26–29 (2001).
Wurtele, M. et al. How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nature Struct. Biol. 8, 23–26 (2001).
Smith, C. L., Khandelwal, P., Keliikuli, K., Zuiderweg, E. R. & Saper, M. A. Structure of the type III secretion and substrate-binding domain of Yersinia YopH phosphatase. Mol. Microbiol. 42, 967–979 (2001).
Sauer, F. et al. Structural basis of chaperone function and pilus biogenesis. Science 285, 1058–1061 (1999).
Evdokimov, A. G., Tropea, J. E., Routzahn, K. M., Copeland, T. D. & Waugh, D. S. Structure of the N-terminal domain of Yersinia pestis YopH at 2.0 Å resolution. Acta Crystallogr. D 57, 793–799 (2001).
Montagna, L. G., Ivanov, M. I. & Bliska, J. B. Identification of residues in the N-terminal domain of the Yersinia tyrosine phosphatase that are critical for substrate recognition. J. Biol. Chem. 276, 5005–5011 (2001).
Young, J. C., Moarefi, I. & Hartl, F. U. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154, 267–273 (2001).
Driessen, A. J. SecB, a molecular chaperone with two faces. Trends Microbiol. 9, 193–196 (2001).
Driessen, A. J., Manting, E. H. & van der Does, C. The structural basis of protein targeting and translocation in bacteria. Nature Struct. Biol. 8, 492–498 (2001).
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Stebbins, C., Galán, J. Priming virulence factors for delivery into the host. Nat Rev Mol Cell Biol 4, 738–744 (2003). https://doi.org/10.1038/nrm1201
Issue date:
DOI: https://doi.org/10.1038/nrm1201
This article is cited by
-
Computational approach to predict species-specific type III secretion system (T3SS) effectors using single and multiple genomes
BMC Genomics (2016)
-
Structural Insights on Two Hypothetical Secretion Chaperones from Xanthomonas axonopodis pv. citri
The Protein Journal (2011)
-
Recombinant protein secretion via the type III secretion system
Korean Journal of Chemical Engineering (2011)
-
Salmonella effector proteins and host-cell responses
Cellular and Molecular Life Sciences (2011)
-
Protein delivery into eukaryotic cells by type III secretion machines
Nature (2006)