Key Points
-
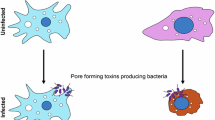
Pore-forming toxins (PFTs), which are expressed as virulence factors by many pathogenic bacteria, and pore-forming proteins (PFPs) have been found in all kingdoms of life
-
PFTs and PFPs undergo a structural and functional metamorphosis from soluble, inactive monomers to active, complex multimeric transmembrane pores that insert into the membranes of target cells
-
Based on their structure and mechanism of pore formation, six families of PFTs and PFPs have been described, each of which has a distinct structure and mechanism of pore formation. These families can be grouped into two larger classes, α-PFTs and β-PFTs (or PFPs), based on the secondary structures of their transmembrane pore domains
-
Owing to substantial recent advances in the structural biology of PFTs, we are beginning to understand the pore architecture and the mechanism of pore formation for all six families
-
The specificity of PFTs and PFPs is determined by their interactions with lipids, sugars and/or protein receptors present in, or on, the target cell membrane
-
Structural modularity enables toxins with the same pore-forming mechanism to target different host cell types by binding to different receptors
-
For PFTs that contribute to infection, examining their structures, dynamics and interactions with host cells at molecular resolution provides cues for the development of therapeutics that could be highly effective in fighting disease
Abstract
Pore-forming toxins (PFTs) are virulence factors produced by many pathogenic bacteria and have long fascinated structural biologists, microbiologists and immunologists. Interestingly, pore-forming proteins with remarkably similar structures to PFTs are found in vertebrates and constitute part of their immune system. Recently, structural studies of several PFTs have provided important mechanistic insights into the metamorphosis of PFTs from soluble inactive monomers to cytolytic transmembrane assemblies. In this Review, we discuss the diverse pore architectures and membrane insertion mechanisms that have been revealed by these studies, and we consider how these features contribute to binding specificity for different membrane targets. Finally, we explore the potential of these structural insights to enable the development of novel therapeutic strategies that would prevent both the establishment of bacterial resistance and an excessive immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Los, F. C., Randis, T. M., Aroian, R. V. & Ratner, A. J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 77, 173–207 (2013).
Bischofberger, M., Iacovache, I. & van der Goot, F. G. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275 (2012).
Alves, G. G., Machado de Avila, R. A., Chavez-Olortegui, C. D. & Lobato, F. C. Clostridium perfringens ε-toxin: the third most potent bacterial toxin known. Anaerobe 30, 102–107 (2014).
Lesieur, C., Vecsey-Semjn, B., Abrami, L., Fivaz, M. & van der Goot, F. G. Membrane insertion: the strategy of toxins. Mol. Membrane Biol. 14, 45–64 (1997).
Iacovache, I., Bischofberger, M. & van der Goot, F. G. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 20, 241–246 (2010).
Gouaux, E. Channel-forming toxins: tales of transformation. Curr. Opin. Struct. Biol. 7, 566–573 (1997).
Szczesny, P. et al. Extending the aerolysin family: from bacteria to vertebrates. PLoS ONE 6, e20349 (2011). This study extended the boundaries of the aerolysin family beyond bacteria to a species range that encompasses all kingdoms of life.
Galinier, R. et al. Biomphalysin, a new β pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni. PLoS Pathog. 9, e1003216 (2013).
Xiang, Y. et al. Host-derived, pore-forming toxin-like protein and trefoil factor complex protects the host against microbial infection. Proc. Natl Acad. Sci. USA 111, 6702–6707 (2014).
Alonzo, F. & Torres, V. J. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 78, 199–230 (2014).
Diabate, M. et al. Escherichia coli α-hemolysin counteracts the anti-virulence innate immune response triggered by the rho GTPase activating toxin CNF1 during bacteremia. PLoS Pathog. 11, e1004732 (2015).
Lakey, J. H., van der Goot, F. G. & Pattus, F. All in the family: the toxic activity of pore-forming toxins. Toxicology 87, 85–108 (1994).
Cascales, E. et al. Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 (2007).
Parker, M. W., Pattus, F., Tucker, A. D. & Tsernoglou, D. Structure of the membrane-pore-forming fragment of colicin A. Nature 337, 93–96 (1989). This article shows the first structure of the soluble form of a PFT, which provided new insights into the mechanism of pore formation.
Lakey, J. H. et al. Membrane insertion of the pore-forming domain of colicin A. A spectroscopic study. Eur. J. Biochem. 196, 599–607 (1991).
Ridley, H., Johnson, C. L. & Lakey, J. H. Interfacial interactions of pore-forming colicins. Adv. Exp. Med. Biol. 677, 81–90 (2010).
Parker, M. W., Tucker, A. D., Tsernoglou, D. & Pattus, F. Insights into membrane insertion based on studies of colicins. Trends Biochem. Sci. 15, 126–129 (1990).
Parker, M. W., Postma, J. P., Pattus, F., Tucker, A. D. & Tsernoglou, D. Refined structure of the pore-forming domain of colicin A at 2.4 Å resolution. J. Mol. Biol. 224, 639–657 (1992).
Kienker, P. K., Qiu, X., Slatin, S. L., Finkelstein, A. & Jakes, K. S. Transmembrane insertion of the colicin Ia hydrophobic hairpin. J. Membr. Biol. 157, 27–37 (1997).
Kim, Y., Valentine, K., Opella, S. J., Schendel, S. L. & Cramer, W. A. Solid-state NMR studies of the membrane-bound closed state of the colicin E1 channel domain in lipid bilayers. Protein Sci. 7, 342–348 (1998).
Tory, M. C. & Merrill, A. R. Adventures in membrane protein topology. A study of the membrane-bound state of colicin E1. J. Biol. Chem. 274, 24539–24549 (1999).
Shin, Y. K., Levinthal, C., Levinthal, F. & Hubbell, W. L. Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. Science 259, 960–963 (1993).
Pulagam, L. P. & Steinhoff, H. J. Acidic pH-induced membrane insertion of colicin A into E. coli natural lipids probed by site-directed spin labeling. J. Mol. Biol. 425, 1782–1794 (2013).
Slatin, S. L., Qiu, X. Q., Jakes, K. S. & Finkelstein, A. Identification of a translocated protein segment in a voltage-dependent channel. Nature 371, 158–161 (1994).
Dunkel, S., Pulagam, L. P., Steinhoff, H. J. & Klare, J. P. In vivo EPR on spin labeled colicin A reveals an oligomeric assembly of the pore-forming domain in E. coli membranes. Phys. Chem. Chem. Phys. 17, 4875–4878 (2015).
Greig, S. L., Radjainia, M. & Mitra, A. K. Oligomeric structure of colicin ia channel in lipid bilayer membranes. J. Biol. Chem. 284, 16126–16134 (2009).
Choe, S. et al. The crystal structure of diphtheria toxin. Nature 357, 216–222 (1992).
Xu, C., Wang, B. C., Yu, Z. & Sun, M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins (Basel) 6, 2732–2770 (2014).
Barta, M. L. et al. The structures of coiled-coil domains from type III secretion system translocators reveal homology to pore-forming toxins. J. Mol. Biol. 417, 395–405 (2012).
Westphal, D., Dewson, G., Czabotar, P. E. & Kluck, R. M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 1813, 521–531 (2011).
Garcia-Saez, A. J., Fuertes, G., Suckale, J. & Salgado, J. Permeabilization of the outer mitochondrial membrane by Bcl-2 proteins. Adv. Exp. Med. Biol. 677, 91–105 (2010).
Hunt, S., Green, J. & Artymiuk, P. J. Hemolysin E (HlyE, ClyA, SheA) and related toxins. Adv. Exp. Med. Biol. 677, 116–126 (2010).
Madegowda, M., Eswaramoorthy, S., Burley, S. K. & Swaminathan, S. X-ray crystal structure of the B component of hemolysin BL from Bacillus cereus. Proteins 71, 534–540 (2008).
Jessberger, N., Dietrich, R., Bock, S., Didier, A. & Martlbauer, E. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 77, 49–57 (2014).
Wallace, A. J. et al. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100, 265–276 (2000).
Mueller, M., Grauschopf, U., Maier, T., Glockshuber, R. & Ban, N. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature 459, 726–730 (2009). This paper reports the first atomic-resolution structure of an α-PFT pore, revealing the complex protomer rearrangement required for pore assembly.
Ganash, M. et al. Structure of the NheA component of the Nhe toxin from Bacillus cereus: implications for function. PLoS ONE 8, e74748 (2013).
Vaidyanathan, M. S., Sathyanarayana, P., Maiti, P. K., Visweswariah, S. S. & Ayappa, K. G. Lysis dynamics and membrane oligomerization pathways for Cytolysin A (ClyA) pore-forming toxin. RSC Adv. 4, 4930–4942 (2014).
Fahie, M. et al. A non-classical assembly pathway of Escherichia coli pore-forming toxin cytolysin A. J. Biol. Chem. 288, 31042–31051 (2013).
Elluri, S. et al. Outer membrane vesicles mediate transport of biologically active Vibrio cholerae cytolysin (VCC) from V. cholerae strains. PLoS ONE 9, e106731 (2014).
Kristan, K. C., Viero, G., Dalla Serra, M., Macek, P. & Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 54, 1125–1134 (2009).
Hinds, M. G., Zhang, W., Anderluh, G., Hansen, P. E. & Norton, R. S. Solution structure of the eukaryotic pore-forming cytolysin equinatoxin II: implications for pore formation. J. Mol. Biol. 315, 1219–1229 (2002).
Athanasiadis, A., Anderluh, G., Macek, P. & Turk, D. Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure 9, 341–346 (2001).
Mancheno, J. M., Martin-Benito, J., Martinez-Ripoll, M., Gavilanes, J. G. & Hermoso, J. A. Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure 11, 1319–1328 (2003).
Mechaly, A. E. et al. Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure 19, 181–191 (2011).
Barlic, A. et al. Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J. Biol. Chem. 279, 34209–34216 (2004).
Ros, U. et al. The sticholysin family of pore-forming toxins induces the mixing of lipids in membrane domains. Biochim. Biophys. Acta 1828, 2757–2762 (2013).
Rojko, N. et al. Membrane damage by an α-helical pore-forming protein, equinatoxin II, proceeds through a succession of ordered steps. J. Biol. Chem. 288, 23704–23715 (2013).
Baker, M. A., Rojko, N., Cronin, B., Anderluh, G. & Wallace, M. I. Photobleaching reveals heterogeneous stoichiometry for equinatoxin II oligomers. Chembiochem 15, 2139–2145 (2014).
Tanaka, K., Caaveiro, J. M., Morante, K., Gonzalez-Manas, J. M. & Tsumoto, K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 6, 6337 (2015). This study shows the importance of sphingomyelin lipids for the integral assembly of the final PFT pore structure.
Schreiber, M. P., Chan, C. M. & Shorr, A. F. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J. Crit. Care 26, 395–401 (2011).
DuMont, A. L. & Torres, V. J. Cell targeting by the Staphylococcus aureus pore-forming toxins: it's not just about lipids. Trends Microbiol. 22, 21–27 (2014).
Savva, C. G. et al. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens. J. Biol. Chem. 288, 3512–3522 (2013).
Keyburn, A. L., Bannam, T. L., Moore, R. J. & Rood, J. I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2, 1913–1927 (2010).
De, S. & Olson, R. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc. Natl Acad. Sci. USA 108, 7385–7390 (2011).
Jayasinghe, L. & Bayley, H. The leukocidin pore: evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. 14, 2550–2561 (2005).
Yamashita, K. et al. Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components. Proc. Natl Acad. Sci. USA 108, 17314–17319 (2011).
Song, L. et al. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1866 (1996). This paper reported the first atomic-resolution structure of a complete PFT pore inserted in a membrane (a β-PFT pore in this case), highlighting the mechanism required for switching from a soluble inactive toxin to an active haemolytic pore.
Yamashita, D. et al. Molecular basis of transmembrane β-barrel formation of staphylococcal pore-forming toxins. Nat. Commun. 5, 4897 (2014).
Olson, R., Nariya, H., Yokota, K., Kamio, Y. & Gouaux, E. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat. Struct. Biol. 6, 134–140 (1999).
Olson, R. & Gouaux, E. Crystal structure of the Vibrio cholerae cytolysin (VCC) pro-toxin and its assembly into a heptameric transmembrane pore. J. Mol. Biol. 350, 997–1016 (2005).
Huyet, J. et al. Structural insights into δ-toxin pore formation. PLoS ONE 8, e66673 (2013).
Paul, K. & Chattopadhyay, K. Pre-pore oligomer formation by Vibrio cholerae cytolysin: insights from a truncated variant lacking the pore-forming pre-stem loop. Biochem. Biophys. Res. Commun. 443, 189–193 (2014).
Iacovache, I., Dal Peraro, M. & van der Goot, F. G. The Comprehensive Sourcebook of Bacterial Protein Toxins (Elsevier Ltd, 2015).
Ballard, J., Sokolov, Y., Yuan, W.-L., Kagan, B. L. & Tweten, R. K. Activation and mechanism of Clostridium septicum α-toxin. Mol. Microbiol. 10, 627–634 (1993).
Opota, O. et al. Monalysin, a novel ss-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality. PLoS Pathog. 7, e1002259 (2011).
Zhao, F. et al. Comprehensive transcriptome profiling and functional analysis of the frog (Bombina maxima) immune system. DNA Res. 21, 1–13 (2013).
Gao, Q. et al. βγ-CAT, a non-lens βγ-crystallin and trefoil factor complex, induces calcium-dependent platelet apoptosis. Thromb. Haemost. 105, 846–854 (2011).
Parker, M. W. et al. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 367, 292–295 (1994). This paper reported the first structure of a β-PFT in its soluble form and an initial model of pore architecture based on low-resolution EM data.
Abrami, L., Fivaz, M. & van Der Goot, F. G. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 8, 168–172 (2000).
Iacovache, I. et al. A rivet model for channel formation by aerolysin-like pore-forming toxins. EMBO J. 25, 457–466 (2006).
Melton, J. A., Parker, M. W., Rossjohn, J., Buckley, J. T. & Tweten, R. K. The identification and structure of the membrane-spanning domain of the Clostridium septicum α-toxin. J. Biol. Chem. 279, 14315–14322 (2004).
Howard, S. P. & Buckley, J. T. Activation of the hole forming toxin aerolysin by extracellular processing. J. Bacteriol. 163, 336–340 (1985).
Iacovache, I. et al. Dual chaperone role of the C-terminal propeptide in folding and oligomerization of the pore-forming toxin aerolysin. PLoS Pathog. 7, e1002135 (2011).
Degiacomi, M. T. et al. Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism. Nat. Chem. Biol. 9, 623–629 (2013). This study used an integrative modelling approach to reveal the architecture of the aerolysin pore at a near-atomic resolution.
Unno, H., Goda, S. & Hatakeyama, T. Hemolytic lectin CEL-III heptamerizes via a large structural transition from α-helices to a β-barrel during the transmembrane pore formation process. J. Biol. Chem. 289, 12805–12812 (2014).
Popoff, M. R. ε-toxin: a fascinating pore-forming toxin. FEBS J. 278, 4602–4615 (2011).
Popoff, M. R. Clostridial pore-forming toxins: powerful virulence factors. Anaerobe 30, 220–238 (2014).
Briggs, D. C. et al. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J. Mol. Biol. 413, 138–149 (2011).
Kitadokoro, K. et al. Crystal structure of Clostridium perfringens enterotoxin displays features of β-pore-forming toxins. J. Biol. Chem. 286, 19549–19555 (2011).
Yelland, T. S. et al. Structure of a C. perfringens enterotoxin mutant in complex with a modified Claudin-2 extracellular loop 2. J. Mol. Biol. 426, 3134–3147 (2014).
Mancheno, J. M., Tateno, H., Goldstein, I. J., Martinez-Ripoll, M. & Hermoso, J. A. Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 280, 17251–17259 (2005).
Sher, D. J. et al. Hydralysins: a new category of β-pore-forming toxins in cnidaria. Characterization and preliminary structure-function analysis. J. Biol. Chem. 280, 22847–22855 (2005).
De Colibus, L. et al. Structures of lysenin reveal a shared evolutionary origin for pore-forming proteins and its mode of sphingomyelin recognition. Structure 20, 1498–1507 (2012).
Hotze, E. M. & Tweten, R. K. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim. Biophys. Acta 1818, 1028–1038 (2012).
Hotze, E. M. et al. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infect. Immun. 81, 216–225 (2013).
Hadders, M. A., Beringer, D. X. & Gros, P. Structure of C8α-MACPF reveals mechanism of membrane attack in complement immune defense. Science 317, 1552–1554 (2007).
Rosado, C. J. et al. A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551 (2007).
Law, R. H. et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468, 447–451 (2010).
Lukoyanova, N. et al. Conformational changes during pore formation by the perforin-related protein pleurotolysin. PLoS Biol. 13, e1002049 (2015).
Roiko, M. S. & Carruthers, V. B. New roles for perforins and proteases in apicomplexan egress. Cell. Microbiol. 11, 1444–1452 (2009).
Deligianni, E. et al. A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell. Microbiol. 15, 1438–1455 (2013).
Xu, Q. et al. Structure of a membrane-attack complex/perforin (MACPF) family protein from the human gut symbiont Bacteroides thetaiotaomicron. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 66, 1297–1305 (2010).
Chatzidaki-Livanis, M., Coyne, M. J. & Comstock, L. E. An antimicrobial protein of the gut symbiont Bacteroides fragilis with a MACPF domain of host immune proteins. Mol. Microbiol. 94, 1361–1374 (2014).
Rossjohn, J., Feil, S. C., McKinstry, W. J., Tweten, R. K. & Parker, M. W. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89, 685–692 (1997). This paper presented the first structure of a CDC (PFO) and a model of the pore, which revealed the mechanism of pore insertion and the role of cholesterol lipids as CDC receptors.
Xu, L. et al. Crystal structure of cytotoxin protein suilysin from Streptococcus suis. Protein Cell 1, 96–105 (2010).
Johnson, S., Brooks, N. J., Smith, R. A., Lea, S. M. & Bubeck, D. Structural basis for recognition of the pore-forming toxin intermedilysin by human complement receptor CD59. Cell Rep. 3, 1369–1377 (2013).
Koster, S. et al. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 5, 3690 (2014).
Feil, S. C. et al. Structure of the lectin regulatory domain of the cholesterol-dependent cytolysin lectinolysin reveals the basis for its lewis antigen specificity. Structure 20, 248–258 (2012).
Bourdeau, R. W. et al. Cellular functions and X-ray structure of anthrolysin O, a cholesterol-dependent cytolysin secreted by Bacillus anthracis. J. Biol. Chem. 284, 14645–14656 (2009).
Feil, S. C., Ascher, D. B., Kuiper, M. J., Tweten, R. K. & Parker, M. W. Structural studies of Streptococcus pyogenes streptolysin O provide insights into the early steps of membrane penetration. J. Mol. Biol. 426, 785–792 (2014).
Tilley, S. J., Orlova, E. V., Gilbert, R. J., Andrew, P. W. & Saibil, H. R. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121, 247–256 (2005).
Czajkowsky, D. M., Hotze, E. M., Shao, Z. & Tweten, R. K. Vertical collapse of a cytolysin prepore moves its transmembrane β-hairpins to the membrane. EMBO J. 23, 3206–3215 (2004).
Shepard, L. A. et al. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry 37, 14563–14574 (1998). This study revealed a structure for the membrane-spanning domain of PFO, and showed the structural switch that accompanies pore formation.
Shatursky, O. et al. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99, 293–299 (1999).
Sato, T. K., Tweten, R. K. & Johnson, A. E. Disulfide-bond scanning reveals assembly state and β-strand tilt angle of the PFO β-barrel. Nat. Chem. Biol. 9, 383–389 (2013).
Ramachandran, R., Tweten, R. K. & Johnson, A. E. Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit β-strand alignment. Nat. Struct. Mol. Biol. 11, 697–705 (2004).
Sonnen, A. F., Plitzko, J. M. & Gilbert, R. J. Incomplete pneumolysin oligomers form membrane pores. Open Biol. 4, 140044 (2014).
Leung, C. et al. Stepwise visualization of membrane pore formation by suilysin, a bacterial cholesterol-dependent cytolysin. eLIFE 3, e04247 (2014).
Wade, K. R. et al. An intermolecular electrostatic interaction controls the prepore-to-pore transition in a cholesterol-dependent cytolysin. Proc. Natl Acad. Sci. USA 112, 2204–2209 (2015).
Reboul, C. F., Whisstock, J. C. & Dunstone, M. A. A new model for pore formation by cholesterol-dependent cytolysins. PLoS Comput. Biol. 10, e1003791 (2014).
Jiang, J., Pentelute, B. L., Collier, R. J. & Zhou, Z. H. Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature 521, 545–549 (2015).
Kintzer, A. F., Sterling, H. J., Tang, I. I., Williams, E. R. & Krantz, B. A. Anthrax toxin receptor drives protective antigen oligomerization and stabilizes the heptameric and octameric oligomer by a similar mechanism. PLoS ONE 5, e13888 (2010).
Krantz, B. A. et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 309, 777–781 (2005).
Meusch, D. et al. Mechanism of Tc toxin action revealed in molecular detail. Nature 508, 61–65 (2014).
Gatsogiannis, C. et al. A syringe-like injection mechanism in Photorhabdus luminescens toxins. Nature 495, 520–523 (2013).
Levan, S., De, S. & Olson, R. Vibrio cholerae cytolysin recognizes the heptasaccharide core of complex N-glycans with nanomolar affinity. J. Mol. Biol. 425, 944–957 (2013).
Rai, A. K., Paul, K. & Chattopadhyay, K. Functional mapping of the lectin activity site on the β-prism domain of Vibrio cholerae cytolysin: implications for the membrane pore-formation mechanism of the toxin. J. Biol. Chem. 288, 1665–1673 (2013).
Kaus, K., Lary, J. W., Cole, J. L. & Olson, R. Glycan specificity of the Vibrio vulnificus hemolysin lectin outlines evolutionary history of membrane targeting by a toxin family. J. Mol. Biol. 426, 2800–2812 (2014).
Hong, Y. et al. Requirement of N-glycan on GPI-anchored proteins for efficient binding of aerolysin but not Clostridium septicum α-toxin. EMBO J. 21, 5047–5056 (2002).
Diep, D. B., Nelson, K. L., Raja, S. M., cMaster, R. W. & Buckley, J. T. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J. Biol. Chem. 273, 2355–2360 (1998).
Cole, A. R. et al. Clostridium perfringens ε-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat. Struct. Mol. Biol. 11, 797–798 (2004).
Akiba, T. et al. Crystallization of parasporin-2, a Bacillus thuringiensis crystal protein with selective cytocidal activity against human cells. Acta Crystallogr. D Biol. Crystallogr. 60, 2355–2357 (2004).
Ivie, S. E. & McClain, M. S. Identification of amino acids important for binding of Clostridium perfringens ε-toxin to host cells and to HAVCR1. Biochemistry 51, 7588–7595 (2012).
Bokori-Brown, M. et al. Clostridium perfringens ε-toxin H149A mutant as a platform for receptor binding studies. Protein Sci. 22, 650–659 (2013).
Shewell, L. K. et al. The cholesterol-dependent cytolysins pneumolysin and streptolysin O require binding to red blood cell glycans for hemolytic activity. Proc. Natl Acad. Sci. USA 111, E5312–5320 (2014).
Johnson, C. L. et al. The antibacterial toxin colicin N binds to the inner core of lipopolysaccharide and close to its translocator protein. Mol. Microbiol. 92, 440–452 (2014).
Mukherjee, S. et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 505, 103–107 (2014).
Fivaz, M., Abrami, L. & van der Goot, F. G. Landing on lipid rafts. Trends Cell Biol. 9, 212–213 (1999).
Abrami, L. & van der Goot, F. G. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J. Cell Biol. 147, 175–184 (1999).
Kobayashi, T., Makino, A., Ishii, K., Yamaji, A. & Kiyokawa, E. Lysenin:sphingomyelin specific probe. Mol Biol Cell Abstr. 11, 314a (2000).
Skocaj, M. et al. The sensing of membrane microdomains based on pore-forming toxins. Curr. Med. Chem. 20, 491–501 (2013).
Lin, Q. & London, E. Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O. J. Biol. Chem. 288, 1340–1352 (2013).
Tweten, R. K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73, 6199–6209 (2005).
Dowd, K. J., Farrand, A. J. & Tweten, R. K. The cholesterol-dependent cytolysin signature motif: a critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 8, e1002787 (2012).
Farrand, A. J., LaChapelle, S., Hotze, E. M., Johnson, A. E. & Tweten, R. K. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl Acad. Sci. USA 107, 4341–4346 (2010).
Alonzo, F. et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 (2013). This study revealed how the selectivity of leukocidins towards different immune cells is mediated by specific chemokine receptors.
Reyes-Robles, T. et al. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14, 453–459 (2013). S. aureus LukED is shown in this study to target both innate and adaptive immune responses by binding to CXCR1 and CXCR2 on neutrophils in addition to its established role of binding to CCR5 on T lymphocytes, macrophages and dendritic cells.
Spaan, A. N. et al. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13, 584–594 (2013).
Spaan, A. N. et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 5, 5438 (2014).
Wilke, G. A. & Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. Proc. Natl Acad. Sci. USA 107, 13473–13478 (2010).
Berube, B. J. & Bubeck Wardenburg, J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel) 5, 1140–1166 (2013).
Giddings, K. S., Zhao, J., Sims, P. J. & Tweten, R. K. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 11, 1173–1178 (2004). This study extended the known cellular specificity of CDCs by showing that they bind to protein receptors such as CD59 in addition to cholesterol.
Tabata, A. et al. The diversity of receptor recognition in cholesterol-dependent cytolysins. Microbiol. Immunol. 58, 155–171 (2014).
Yang, W. S. et al. Suicide cancer gene therapy using pore-forming toxin, streptolysin O. Mol. Cancer Ther. 5, 1610–1619 (2006).
Ayub, M., Stoddart, D. & Bayley, H. Nucleobase recognition by truncated α-hemolysin pores. ACS Nano 9, 7895–7903 (2015).
Stoddart, D. et al. Functional truncated membrane pores. Proc. Natl Acad. Sci. USA 111, 2425–2430 (2014).
Dong, J. et al. Oroxylin A inhibits hemolysis via hindering the self-assembly of α-hemolysin heptameric transmembrane pore. PLoS Comput. Biol. 9, e1002869 (2013).
Qiu, J. et al. Molecular modeling reveals the novel inhibition mechanism and binding mode of three natural compounds to staphylococcal α-hemolysin. PLoS ONE 8, e80197 (2013).
Vivekananda, J., Salgado, C. & Millenbaugh, N. J. DNA aptamers as a novel approach to neutralize Staphylococcus aureus α-toxin. Biochem. Biophys. Res. Commun. 444, 433–438 (2014).
Rai, A. K. & Chattopadhyay, K. Trapping of Vibrio cholerae cytolysin in the membrane-bound monomeric state blocks membrane insertion and functional pore formation by the toxin. J. Biol. Chem. 289, 16978–16987 (2014).
Wu, Q. & Guo, Z. Glycosylphosphatidylinositols are potential targets for the development of novel inhibitors for aerolysin-type of pore-forming bacterial toxins. Med. Res. Rev. 30, 258–269 (2010).
Foletti, D. et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J. Mol. Biol. 425, 1641–1654 (2013).
Inoshima, I. et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314 (2011).
Fernandes da Costa, S. P. et al. Identification of a key residue for oligomerisation and pore-formation of Clostridium perfringens NetB. Toxins (Basel) 6, 1049–1061 (2014).
Bokori-Brown, M. et al. Clostridium perfringens ε toxin mutant Y30A-Y196A as a recombinant vaccine candidate against enterotoxemia. Vaccine 32, 2682–2687 (2014).
Cockeran, R. et al. Characterization of the interactions of the pneumolysoid, Δ6 PLY, with human neutrophils in vitro. Vaccine 29, 8780–8782 (2011).
Douce, G., Ross, K., Cowan, G., Ma, J. T. & Mitchell, T. J. Novel mucosal vaccines generated by genetic conjugation of heterologous proteins to pneumolysin (PLY) from Streptococcus pneumoniae. Vaccine 28, 3231–3237 (2010).
Mann, B. et al. Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid-CbpA peptide recombinant fusion protein. J. Infect. Dis. 209, 1116–1125 (2014).
Hu, C. M. & Zhang, L. Nanotoxoid vaccines. Nano Today 9, 401–404 (2014).
Hu, C. M., Fang, R. H., Luk, B. T. & Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 8, 933–938 (2013). This study used Hla pores embedded in membrane-coated nanoparticles to promote an enhanced toxin-specific immune response.
Walther, W. et al. Novel Clostridium perfringens enterotoxin suicide gene therapy for selective treatment of claudin-3- and -4-overexpressing tumors. Gene Ther. 19, 494–503 (2012).
Lal-Nag, M., Battis, M., Santin, A. D. & Morin, P. J. Claudin-6: a novel receptor for CPE-mediated cytotoxicity in ovarian cancer. Oncogenesis 1, e33 (2012).
Veshnyakova, A. et al. Mechanism of Clostridium perfringens enterotoxin interaction with Claudin-3/-4 protein suggests structural modifications of the toxin to target specific claudins. J. Biol. Chem. 287, 1698–1708 (2012).
Linhartova, I. et al. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34, 1076–1112 (2010).
Kudryashova, E., Heisler, D., Zywiec, A. & Kudryashov, D. S. Thermodynamic properties of the effector domains of MARTX toxins suggest their unfolding for translocation across the host membrane. Mol. Microbiol. 92, 1056–1071 (2014).
Hyland, C., Vuillard, L., Hughes, C. & Koronakis, V. Membrane interaction of Escherichia coli hemolysin: flotation and insertion-dependent labeling by phospholipid vesicles. J. Bacteriol. 183, 5364–5370 (2001).
Gonzalez, M. R. et al. Pore-forming toxins induce multiple cellular responses promoting survival. Cell. Microbiol. 13, 1026–1043 (2011).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006).
Higa, N. et al. Vibrio parahaemolyticus effector proteins suppress inflammasome activation by interfering with host autophagy signaling. PLoS Pathog. 9, e1003142 (2013).
Nagahama, M. et al. The p38 MAPK and JNK pathways protect host cells against Clostridium perfringens β-toxin. Infect. Immun. 81, 3703–3708 (2013).
Craven, R. R. et al. Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE 4, e7446 (2009).
Soong, G., Chun, J., Parker, D. & Prince, A. S. aureus activation of caspase-1/calpain signaling mediates invasion through human keratinocytes. J. Infect. Dis. 205, 1571–1579 (2012).
Holzinger, D. et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 92, 1069–1081 (2012).
Di Venanzio, G., Stepanenko, T. M. & Garcia Vescovi, E. Serratia marcescens ShlA pore-forming toxin is responsible for early induction of autophagy in host cells and is transcriptionally regulated by RcsB. Infect. Immun. 82, 3542–3554 (2014).
Mestre, M. B. & Colombo, M. I. Autophagy and toxins: a matter of life or death. Curr. Mol. Med. 13, 241–251 (2013).
Mestre, M. B. & Colombo, M. I. Staphylococcus aureus promotes autophagy by decreasing intracellular cAMP levels. Autophagy 8, 1865–1867 (2012).
Hamon, M. A. et al. Histone modifications induced by a family of bacterial toxins. Proc. Natl Acad. Sci. USA 104, 13467–13472 (2007).
Walev, I. et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl Acad. Sci. USA 98, 3185–3190 (2001).
Keefe, D. et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 23, 249–262 (2005).
McNeil, P. L. & Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6, 499–505 (2005).
Lesieur, C. et al. Increased stability upon heptamerization of the pore-forming toxin aerolysin. J. Biol. Chem. 274, 36722–36728 (1999).
Idone, V., Tam, C. & Andrews, N. W. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 18, 552–559 (2008).
Husmann, M. et al. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 583, 337–344 (2009).
Corrotte, M. et al. Caveolae internalization repairs wounded cells and muscle fibers. eLIFE 2, e00926 (2013).
Keyel, P. A. et al. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 124, 2414–2423 (2011).
Jimenez, A. J. et al. ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 (2014).
Henne, W. M., Buchkovich, N. J. & Emr, S. D. The ESCRT pathway. Dev. Cell 21, 77–91 (2011).
Strack, B., Calistri, A., Craig, S., Popova, E. & Gottlinger, H. G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114, 689–699 (2003).
Prescher, J. et al. Super-resolution imaging of ESCRT-proteins at HIV-1 assembly sites. PLoS Pathog. 11, e1004677 (2015).
Degiacomi, M. T. & Dal Peraro, M. Macromolecular symmetric assembly prediction using swarm intelligence dynamic modeling. Structure 21, 1097–1106 (2013).
Tamo, G. E., Abriata, L. A. & Dal Peraro, M. The importance of dynamics in integrative modeling of supramolecular assemblies. Curr. Opin. Struct. Biol. 31, 28–34 (2015).
Spiga, E., Degiacomi, M. T. & Dal Peraro, M. New strategies for integrative dynamic modeling of macromolecular assembly. Adv. Protein Chem. Struct. Biol. 96, 77–111 (2014).
Russel, D. et al. Putting the pieces together: integrative modeling platform software for structure determination of macromolecular assemblies. PLoS Biol. 10, e1001244 (2012).
Thalassinos, K., Pandurangan, A. P., Xu, M., Alber, F. & Topf, M. Conformational states of macromolecular assemblies explored by integrative structure calculation. Structure 21, 1500–1508 (2013).
Kudryashev, M. et al. In situ structural analysis of the Yersinia enterocolitica injectisome. eLIFE 2, e00792 (2013).
Sali, A. et al. Outcome of the first wwPDB hybrid/integrative methods task force workshop. Structure 23, 1156–1167 (2015).
Leone, P. et al. X-ray and cryo-electron microscopy structures of monalysin pore-forming toxin reveal multimerization of the pro-form. J. Biol. Chem. 290, 13191–13201 (2015).
Acknowledgements
Work in the authors' laboratories is supported by the Swiss National Science Foundation (SNSF). The authors apologize to colleagues whose work could not be duly discussed owing to space limitations. The authors thank M. Dunstone for providing the coordinates of perfringolysin O oligomers used in the figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Lipid droplets
-
Cellular organelles that store metabolic energy in the form of neutral lipids, such as triglycerides. These neutral lipids form the core of the droplet, which is surrounded by a phospholipid monolayer.
- Exosomes
-
Vesicles that are released into the extracellular space from the lumen of multivesicular bodies.
- Caveolae
-
Surface invaginations that may pinch off to allow cellular uptake of extracellular material.
- Multivesicular bodies
-
Late endocytic organelles that contain intraluminal vesicles that are formed by inward invagination of the limiting membrane.
- Programmed necrosis
-
A form of necrosis that is mediated by regulated pathways.
- Pyroptosis
-
A caspase 1-dependent form of programmed cell death that occurs as an antimicrobial response.
- Electron paramagnetic resonance
-
(EPR). A spectroscopy technique used to study paramagnetic molecules (that is, molecules with unpaired electrons). In biology, paramagnetic spin labels are covalently added to protein complexes to extract low-resolution information about their structure and dynamics.
- Outer membrane vesicles
-
(OMVs). Vesicles that are derived from the outer membrane of Gram-negative bacteria.
- Sphingomyelin
-
A sphingolipid found in animal cells that generally has a phosphocholine headgroup.
- Phase-separated lipid membranes
-
Membranes within which lipids are separated into different domains.
- Differential scanning calorimetry
-
A technique used to characterize the energetics associated with conformational changes of biomolecules, such as protein folding or phase transitions in lipid and lipid–protein mixtures upon temperature variation.
- Atomic force microscopy
-
(AFM). A technique that uses the deflection of a sharp-tipped probe to measure the local conformation and mechanical properties of a sample (for example, proteins embedded in a membrane) with up to nanometre resolution.
- Lectin
-
One of a family of proteins that bind to sugar moieties in glycoproteins.
- Parasitophorous vacuole
-
The endosome-like organelle in which parasites reside upon engulfment by the target cell.
- Disulfide scanning
-
An approach in which each amino acid in a sequence of interest is sequentially mutated to cysteine using a single point mutation. The reactivity of the introduced cysteine is analysed, for example, using a functional assay, to assess the dynamic location of the amino acid in the protein structure.
- Translocon
-
A protein channel that enables the translocation of client proteins across a membrane.
- GPI anchor
-
A glycosylphosphatidylinositol (GPI) lipid that is covalently linked to the carboxy terminus of a peripheral protein. The anchor attaches the protein to the outer leaflet of the plasma membrane.
- Lipid rafts
-
Microscale or nanoscale domains of biological membranes that are rich in cholesterol and sphingolipids.
- DNA aptamers
-
Short oligonucleotides engineered and selected to specifically bind to target molecules with high affinity. As with antibodies (their protein counterparts), DNA aptamers have broad applications both in biotechnology and therapeutics.
- Toxoids
-
Bacterial toxins engineered to decrease virulence. Toxoids are used as vaccines for microbial infections as they contribute to the development of an immune response against the native toxin.
Rights and permissions
About this article
Cite this article
Peraro, M., van der Goot, F. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14, 77–92 (2016). https://doi.org/10.1038/nrmicro.2015.3
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro.2015.3
This article is cited by
-
Bioinspired nanomaterials for the treatment of bacterial infections
Nano Research (2024)
-
Surveying membrane landscapes: a new look at the bacterial cell surface
Nature Reviews Microbiology (2023)
-
Glucocorticoids increase tissue cell protection against pore-forming toxins from pathogenic bacteria
Communications Biology (2023)
-
Bacterial toxins and heart function: heat-labile Escherichia coli enterotoxin B promotes changes in cardiac function with possible relevance for sudden cardiac death
Biophysical Reviews (2023)
-
Visualizing the Domino-Like Prepore-to-Pore Transition of Streptolysin O by High-Speed AFM
The Journal of Membrane Biology (2023)