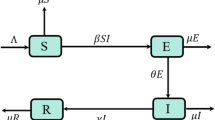
Key Points
-
Norovirus infections pose a substantial risk to human health worldwide. Modes of viral transmission, the severity of illness and evolutionary pressures all contribute to this risk and can vary between viral genotypes.
-
Many details about the transmission of noroviruses remain unknown, especially regarding the origin of newly emerging strains.
-
The recent emergence of genotype GII.P17-GII.17 noroviruses in Asia should serve as a warning that future risks from norovirus outbreaks might arise from genotypes other than those currently targeted by vaccine development.
-
Bacteria in the host microbiota might influence human norovirus infections by providing HBGA-like sugars for norovirus attachment and by modulating host immunity.
-
B cells support norovirus replication in the presence of bacteria that express histo-blood group antigen (HBGA)-like sugars. A recently described cell culture system for the study of noroviruses in B cells will hopefully advance our understanding of many aspects of human noroviruses, ranging from the molecular characterization of their life cycle to the development of improved vaccines.
Abstract
Norovirus infections are a major cause of gastroenteritis, and outbreaks occur frequently. Several factors are currently increasing the challenge posed by norovirus infections to global health, notably the increasing number of infections in immunocompromised individuals, who are more susceptible to disease, and the globalization of the food industry, which enables large norovirus outbreaks to occur on an international scale. Furthermore, the rapid rate of the genetic and antigenic evolution of circulating noroviruses complicates the development of vaccines and therapies that are required to counter these challenges. In this Review, we describe recent advances in the study of the transmission, pathogenesis and evolution of human noroviruses, and consider the ongoing risk of norovirus outbreaks, together with the future prospects for therapeutics, in a rapidly changing world.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Atmar, R. L. & Estes, M. K. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. North Am. 35, 275–290 (2006).
Atmar, R. L. et al. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14, 1553–1557 (2008).
Teunis, P. F. et al. Norwalk virus: how infectious is it? J. Med. Virol. 80, 1468–1476 (2008).
Marks, P. J. et al. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 124, 481–487 (2000).
Verhoef, L. et al. Norovirus genotype profiles associated with foodborne transmission, 1999–2012. Emerg. Infect. Dis. 21, 592–599 (2015).
Wikswo, M. E. & Hall, A. J. Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. MMWR Surveill. Summ. 61, 1–12 (2012).
Lysen, M. et al. Genetic diversity among food-borne and waterborne norovirus strains causing outbreaks in Sweden. J. Clin. Microbiol. 47, 2411–2418 (2009).
Siebenga, J. J. et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J. Infect. Dis. 200, 802–812 (2009).
Kroneman, A. et al. Data quality of 5 years of central norovirus outbreak reporting in the European Network for food-borne viruses. J. Public Health (Oxf) 30, 82–90 (2008).
Harris, J. P., Edmunds, W. J., Pebody, R., Brown, D. W. & Lopman, B. A. Deaths from norovirus among the elderly, England and Wales. Emerg. Infect. Dis. 14, 1546–1552 (2008).
Trivedi, T. K. et al. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am. J. Infect. Control 41, 654–657 (2013).
Siebenga, J. J. et al. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J. Infect. Dis. 198, 994–1001 (2008). A study showing that noroviruses more commonly cause prolonged periods of gastrointestinal illness and virus shedding than previously recognized.
Murata, T. et al. Prolonged norovirus shedding in infants ≤6 months of age with gastroenteritis. Pediatr. Infect. Dis. J. 26, 46–49 (2007).
Jones, M. K. et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346, 755–759 (2014). An important paper that describes a cell culture system for the study of human norovirus infection in B cells.
Vinje, J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 53, 373–381 (2015).
Kroneman, A. et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J. Clin. Microbiol. 46, 2959–2965 (2008).
de Graaf, M. et al. Emergence of a novel GII.17 norovirus — end of the GII.4 era? Euro Surveill. 20, 21178 (2015).
Thorne, L. G. & Goodfellow, I. G. Norovirus gene expression and replication. J. Gen. Virol. 95, 278–291 (2014).
Donaldson, E. F., Lindesmith, L. C., Lobue, A. D. & Baric, R. S. Viral shape-shifting: norovirus evasion of the human immune system. Nat. Rev. Microbiol. 8, 231–241 (2010).
Vongpunsawad, S., Venkataram Prasad, B. V. & Estes, M. K. Norwalk virus minor capsid protein VP2 associates within the VP1 shell domain. J. Virol. 87, 4818–4825 (2013).
Debbink, K., Lindesmith, L. C. & Baric, R. S. The state of norovirus vaccines. Clin. Infect. Dis. 58, 1746–1752 (2014).
Wobus, C. E. et al. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2, e432 (2004).
Hoa Tran, T. N., Trainor, E., Nakagomi, T., Cunliffe, N. A. & Nakagomi, O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J. Clin. Virol. 56, 185–193 (2013).
Rodriguez-Lazaro, D. et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 36, 786–814 (2012).
Le Guyader, F. S., Atmar, R. L. & Le Pendu, J. Transmission of viruses through shellfish: when specific ligands come into play. Curr. Opin. Virol. 2, 103–110 (2012).
Verhoef, L. et al. An integrated approach to identifying international foodborne norovirus outbreaks. Emerg. Infect. Dis. 17, 412–418 (2011).
Foodborne Disease Burden Epidemiology Reference Group 2007–2015. WHO estimates of the global burden of foodborne diseases (WHO, 2011).
Ahmed, S. M. et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 725–730 (2014).
Rockx, B. et al. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35, 246–253 (2002).
Sukhrie, F. H. et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin. Infect. Dis. 54, 931–937 (2012).
Sukhrie, F. H., Siebenga, J. J., Beersma, M. F. & Koopmans, M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J. Clin. Microbiol. 48, 4303–4305 (2010).
Milbrath, M. O., Spicknall, I. H., Zelner, J. L., Moe, C. L. & Eisenberg, J. N. Heterogeneity in norovirus shedding duration affects community risk. Epidemiol. Infect. 141, 1572–1584 (2013).
Debbink, K. et al. Within-host evolution results in antigenically distinct GII.4 noroviruses. J. Virol. 88, 7244–7255 (2014).
Mattison, K. et al. Human noroviruses in swine and cattle. Emerg. Infect. Dis. 13, 1184–1188 (2007).
Summa, M., von Bonsdorff, C. H. & Maunula, L. Pet dogs — a transmission route for human noroviruses? J. Clin. Virol. 53, 244–247 (2012).
Souza, M., Azevedo, M. S., Jung, K., Cheetham, S. & Saif, L. J. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J. Virol. 82, 1777–1786 (2008).
Cheetham, S. et al. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 80, 10372–10381 (2006).
Caddy, S. L. et al. Evidence for human norovirus infection of dogs in the United Kingdom. J. Clin. Microbiol. 53, 1873–1883 (2015).
Widdowson, M. A. et al. Detection of serum antibodies to bovine norovirus in veterinarians and the general population in the Netherlands. J. Med. Virol. 76, 119–128 (2005).
Mesquita, J. R. et al. Presence of antibodies against genogroup VI norovirus in humans. Virol. J. 10, 176 (2013).
Friesema, I. H. et al. Differences in clinical presentation between norovirus genotypes in nursing homes. J. Clin. Virol. 46, 341–344 (2009).
Thorven, M. et al. A homozygous nonsense mutation (428G→A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J. Virol. 79, 15351–15355 (2005).
Lindesmith, L. et al. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9, 548–553 (2003).
Stanley, P. & Cummings, R. D. in Essentials of Glycobiology 2nd edn Ch. 13 (eds Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2009).
Le Pendu, J., Ruvoen-Clouet, N., Kindberg, E. & Svensson, L. Mendelian resistance to human norovirus infections. Semin. Immunol. 18, 375–386 (2006).
Currier, R. L. et al. Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clin. Infect. Dis. 60, 1631–1638 (2015).
Atmar, R. L. Noroviruses — state of the art. Food Environ. Virol. 2, 117–126 (2010).
Shirato, H. et al. Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding. J. Virol. 82, 10756–10767 (2008).
Hutson, A. M., Atmar, R. L., Graham, D. Y. & Estes, M. K. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185, 1335–1337 (2002).
Frenck, R. et al. Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J. Infect. Dis. 206, 1386–1393 (2012).
Donaldson, E. F., Lindesmith, L. C., Lobue, A. D. & Baric, R. S. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol. Rev. 225, 190–211 (2008).
Tan, M. & Jiang, X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 6, e1000983 (2010).
Taube, S. et al. Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J. Virol. 86, 5584–5593 (2012).
Zakhour, M. et al. The αGal epitope of the histo-blood group antigen family is a ligand for bovine norovirus Newbury2 expected to prevent cross-species transmission. PLoS Pathog. 5, e1000504 (2009).
Caddy, S., Breiman, A., le Pendu, J. & Goodfellow, I. Genogroup IV and VI canine noroviruses interact with histo-blood group antigens. J. Virol. 88, 10377–10391 (2014).
Murakami, K. et al. Norovirus binding to intestinal epithelial cells is independent of histo-blood group antigens. PLoS ONE 8, e66534 (2013).
Yazawa, S. et al. Blood group substances as potential therapeutic agents for the prevention and treatment of infection with noroviruses proving novel binding patterns in human tissues. PLoS ONE 9, e89071 (2014).
Chan, M. C., Ho, W. S. & Sung, J. J. In vitro whole-virus binding of a norovirus genogroup II genotype 4 strain to cells of the lamina propria and Brunner's glands in the human duodenum. J. Virol. 85, 8427–8430 (2011).
Duizer, E. et al. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85, 79–87 (2004).
Taube, S. et al. A mouse model for human norovirus. mBio 4, e00450-13 (2013). A paper that describes the first mouse model for human norovirus infection.
Bok, K. et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc. Natl Acad. Sci. USA 108, 325–330 (2011).
Chachu, K. A., LoBue, A. D., Strong, D. W., Baric, R. S. & Virgin, H. W. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4, e1000236 (2008).
Karst, S. M., Wobus, C. E., Lay, M., Davidson, J. & Virgin, H. W. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299, 1575–1578 (2003).
Bui, T. et al. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J. Gen. Virol. 94, 2005–2016 (2013).
Jung, K. et al. The effects of simvastatin or interferon-α on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS ONE 7, e41619 (2012).
Parrino, T. A., Schreiber, D. S., Trier, J. S., Kapikian, A. Z. & Blacklow, N. R. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 297, 86–89 (1977).
Ayukekbong, J. A. et al. Pattern of circulation of norovirus GII strains during natural infection. J. Clin. Microbiol. 52, 4253–4259 (2014).
Simmons, K., Gambhir, M., Leon, J. & Lopman, B. Duration of immunity to norovirus gastroenteritis. Emerg. Infect. Dis. 19, 1260–1267 (2013).
Lindesmith, L. C. et al. Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial. PLoS Med. 12, e1001807 (2015).
Reeck, A. et al. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202, 1212–1218 (2010).
Ramani, S. et al. Mucosal and cellular immune responses to Norwalk virus. J. Infect. Dis. 212, 397–405 (2015).
Yi, W. et al. Escherichia coli O86 O-antigen biosynthetic gene cluster and stepwise enzymatic synthesis of human blood group B antigen tetrasaccharide. J. Am. Chem. Soc. 127, 2040–2041 (2005).
Rasko, D. A., Wang, G., Monteiro, M. A., Palcic, M. M. & Taylor, D. E. Synthesis of mono- and di-fucosylated type I Lewis blood group antigens by Helicobacter pylori. Eur. J. Biochem. 267, 6059–6066 (2000).
Ruvoen-Clouet, N. et al. Increase in genogroup II.4 norovirus host spectrum by CagA-positive Helicobacter pylori infection. J. Infect. Dis. 210, 183–191 (2014).
Baldridge, M. T. et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 347, 266–269 (2015). The first demonstration of the role of the bacterial microbiota in the persistence of enteric norovirus infections.
Li, L. et al. The fecal viral flora of California sea lions. J. Virol. 85, 9909–9917 (2011).
Wu, Z. et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 10, 609–620 (2015).
Katayama, K. et al. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299, 225–239 (2002).
Siebenga, J. J. et al. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog. 6, e1000884 (2010).
Bull, R. A., Eden, J. S., Rawlinson, W. D. & White, P. A. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 6, e1000831 (2010).
Eden, J. S. et al. The emergence and evolution of the novel epidemic norovirus GII.4 variant Sydney 2012. Virology 450–451, 106–113 (2014).
Lopman, B. et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 363, 682–688 (2004).
Debbink, K. et al. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J. Infect. Dis. 208, 1877–1887 (2013).
Lindesmith, L. C. et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5, e31 (2008).
Boon, D. et al. Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J. Virol. 85, 8656–8666 (2011).
Kobayashi, M. et al. Molecular evolution of the capsid gene in norovirus genogroup I. Sci. Rep. 5, 13806 (2015).
Iritani, N. et al. Genetic analysis of the capsid gene of genotype GII.2 noroviruses. J. Virol. 82, 7336–7345 (2008).
Swanstrom, J., Lindesmith, L. C., Donaldson, E. F., Yount, B. & Baric, R. S. Characterization of blockade antibody responses in GII.2.1976 Snow Mountain virus-infected subjects. J. Virol. 88, 829–837 (2014).
Bull, R. A. et al. Norovirus recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 11, 1079–1085 (2005).
Eden, J. S., Tanaka, M. M., Boni, M. F., Rawlinson, W. D. & White, P. A. Recombination within the pandemic norovirus GII.4 lineage. J. Virol. 87, 6270–6282 (2013).
Mahar, J. E., Bok, K., Green, K. Y. & Kirkwood, C. D. The importance of intergenic recombination in norovirus GII.3 evolution. J. Virol. 87, 3687–3698 (2013).
Chang, K. O., Sosnovtsev, S. V., Belliot, G., King, A. D. & Green, K. Y. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353, 463–473 (2006).
Belliot, G. et al. Norovirus proteinase–polymerase and polymerase are both active forms of RNA-dependent RNA polymerase. J. Virol. 79, 2393–2403 (2005).
Subba-Reddy, C. V., Goodfellow, I. & Kao, C. C. VPg-primed RNA synthesis of norovirus RNA-dependent RNA polymerases by using a novel cell-based assay. J. Virol. 85, 13027–13037 (2011).
Subba-Reddy, C. V., Yunus, M. A., Goodfellow, I. G. & Kao, C. C. Norovirus RNA synthesis is modulated by an interaction between the viral RNA-dependent RNA polymerase and the major capsid protein, VP1. J. Virol. 86, 10138–10149 (2012).
Gairard-Dory, A. C. et al. Clinical usefulness of oral immunoglobulins in lung transplant recipients with norovirus gastroenteritis: a case series. Transplant. Proc. 46, 3603–3605 (2014).
Florescu, D. F., Hill, L. A., McCartan, M. A. & Grant, W. Two cases of Norwalk virus enteritis following small bowel transplantation treated with oral human serum immunoglobulin. Pediatr. Transplant. 12, 372–375 (2008).
Chachu, K. A. et al. Antibody is critical for the clearance of murine norovirus infection. J. Virol. 82, 6610–6617 (2008).
Green, K. Y. Norovirus infection in immunocompromised hosts. Clin. Microbiol. Infect. 20, 717–723 (2014).
Rossignol, J. F. & El-Gohary, Y. M. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment. Pharmacol. Ther. 24, 1423–1430 (2006).
Siddiq, D. M., Koo, H. L., Adachi, J. A. & Viola, G. M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 63, 394–397 (2011).
Woodward, J. M. et al. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am. J. Gastroenterol. 110, 320–327 (2015).
Nice, T. J. et al. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273 (2015).
Chang, K. O. & George, D. W. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J. Virol. 81, 12111–12118 (2007).
Richardson, C., Bargatze, R. F., Goodwin, R. & Mendelman, P. M. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev. Vaccines 12, 155–167 (2013).
Bernstein, D. I. et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J. Infect. Dis. 211, 870–878 (2015).
Kocher, J. et al. Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs. J. Virol. 88, 9728–9743 (2014).
Tamminen, K., Lappalainen, S., Huhti, L., Vesikari, T. & Blazevic, V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS ONE 8, e70409 (2013).
van der Vries, E. et al. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS Pathog. 9, e1003343 (2013).
Huynen, P. et al. Molecular epidemiology of norovirus infections in symptomatic and asymptomatic children from Bobo Dioulasso, Burkina Faso. J. Clin. Virol. 58, 515–521 (2013).
Hickman, D. et al. The effect of malnutrition on norovirus infection. mBio 5, e01032-13 (2014).
Frange, P. et al. Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J. Infect. Dis. 206, 1269–1274 (2012).
Meeroff, J. C., Schreiber, D. S., Trier, J. S. & Blacklow, N. R. Abnormal gastric motor function in viral gastroenteritis. Ann. Intern. Med. 92, 370–373 (1980).
Schwartz, S. et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117, 5850–5856 (2011).
Schreiber, D. S., Blacklow, N. R. & Trier, J. S. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J. Infect. Dis. 129, 705–708 (1974).
Troeger, H. et al. Structural and functional changes of the duodenum in human norovirus infection. Gut 58, 1070–1077 (2009).
Desai, R. et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin. Infect. Dis. 55, 189–193 (2012).
Huhti, L. et al. Norovirus GII-4 causes a more severe gastroenteritis than other noroviruses in young children. J. Infect. Dis. 203, 1442–1444 (2011).
Chan, M. C. et al. Fecal viral load and norovirus-associated gastroenteritis. Emerg. Infect. Dis. 12, 1278–1280 (2006).
Kroneman, A. et al. Proposal for a unified norovirus nomenclature and genotyping. Arch. Virol. 158, 2059–2068 (2013).
Acknowledgements
This work was supported by the European Union's Horizon 2020 grant to the COMPARE Consortium, under grant agreement number 643476, and by the Virgo Consortium, funded by the Dutch Government (project number FES0908). The authors thank J. Le Pendu for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Genogroups
-
Groups of related viruses within a genus.
- Polyprotein
-
A large protein that is cleaved into separate smaller proteins with different biological functions.
- Nosocomial transmission
-
The transmission of an infectious disease in a hospital setting.
- Herd immunity
-
General immunity of a host population to a pathogen, mediated by immunity acquired by a high proportion of individuals within the population.
- Gnotobiotic
-
Pertaining to an animal: germ-free, or having known associated microorganisms.
- Lewis blood group
-
A blood group system based on the expression of glycoproteins called Lewis antigens.
- Mesoamerican
-
From a region that extends south and east from central Mexico to include parts of Guatemala, Belize, Honduras and Nicaragua.
- Villous blunting
-
Flattening of the intestinal villi as a result of damage or injury.
- Host cell tropism
-
The specificity of a virus for a particular host cell type.
- Virus-like particles
-
(VLPs). Particles that resemble natural viral particles and contain viral structural proteins, such as the envelope or capsid protein, but do not contain genetic material from the virus.
- Goblet cells
-
Specialized epithelial cells that secrete mucus and are found in the mucous membranes of the stomach, intestines and respiratory passages.
- Lamina propria
-
The layer of connective tissue that underlies the epithelium of a mucous membrane.
- Brunner's glands
-
Tubular submucosal glands found in the duodenum.
- DC-SIGN
-
(Dendritic-cell-specific ICAM3-grabbing non-integrin). A C-type lectin receptor present on the surface of macrophages and dendritic cells.
- Interferon
-
One of several signalling proteins that are crucial components of the innate immune response to viral infection. Many viruses have proteins that block or modulate interferons.
- Phylodynamic reconstruction
-
The reconstruction of epidemiological, immunological and evolutionary processes based on analyses of viral phylogenies.
- Genetic drift
-
The change in the genetic composition of a population that occurs by chance or random events rather than by natural selection.
- Replicon systems
-
Systems in which an incomplete viral genome is capable of autonomous replication.
- Heterologous cross-protection
-
Protection conferred on a host by inoculation with one strain of a microorganism (or a component of the strain) that prevents infection when the host is later challenged with a different strain.
Rights and permissions
About this article
Cite this article
de Graaf, M., van Beek, J. & Koopmans, M. Human norovirus transmission and evolution in a changing world. Nat Rev Microbiol 14, 421–433 (2016). https://doi.org/10.1038/nrmicro.2016.48
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro.2016.48
This article is cited by
-
Comprehensive full genome analysis of norovirus strains from eastern India, 2017–2021
Gut Pathogens (2024)
-
Effect of Human Adenovirus Type 35 Concentration on Its Inactivation and Sorption on Titanium Dioxide Nanoparticles
Food and Environmental Virology (2024)
-
Persistence of Infectious Human Norovirus in Estuarine Water
Food and Environmental Virology (2024)
-
Recovery and Quantification of Norovirus in Air Samples from Experimentally Produced Aerosols
Food and Environmental Virology (2024)
-
The Inhibitory Effect of Resveratrol from Reynoutria japonica on MNV-1, a Human Norovirus Surrogate
Food and Environmental Virology (2024)