Key Points
-
The pili of Gram-negative bacteria are long extracellular polymers that mediate diverse functions, such as bacterial attachment, movement and substrate transport.
-
There are five classes of pili in Gram-negative bacteria: chaperone–usher pili, type IV pili, type IV secretion pili, type V pili and curli fibres.
-
Pili are assembled by multiprotein machineries that are either located in the outer membrane (chaperone–usher pili, type V pili and curli fibres) or span both the inner membrane and outer membrane (type IV pili and type IV conjugative pili).
-
Both chaperone–usher pili and type V pili use a strand exchange mechanism for assembly, whereas the double-membrane-spanning assembly platforms that assemble type IV pili and type IV conjugative pili are powered by cytoplasmic hexameric ATPases that hydrolyse ATP.
-
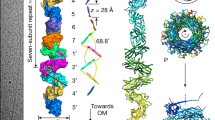
Recent high-resolution structures have provided a detailed picture of the pilus filament of chaperone–usher pili, type IV pili and type IV conjugative pili, which revealed distinct properties.
-
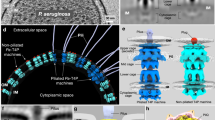
Recent cryo-electron tomography studies have revealed the structure of fully assembled pilus systems in their native environment in the bacterial cell.
-
These pilus systems could provide targets that can be used for the development of novel antibacterial compounds.
Abstract
Pili are crucial virulence factors for many Gram-negative pathogens. These surface structures provide bacteria with a link to their external environments by enabling them to interact with, and attach to, host cells, other surfaces or each other, or by providing a conduit for secretion. Recent high-resolution structures of pilus filaments and the machineries that produce them, namely chaperone–usher pili, type IV pili, conjugative type IV secretion pili and type V pili, are beginning to explain some of the intriguing biological properties that pili exhibit, such as the ability of chaperone–usher pili and type IV pili to stretch in response to external forces. By contrast, conjugative pili provide a conduit for the exchange of genetic information, and recent high-resolution structures have revealed an integral association between the pilin subunit and a phospholipid molecule, which may facilitate DNA transport. In addition, progress in the area of cryo-electron tomography has provided a glimpse of the overall architecture of the type IV pilus machinery. In this Review, we examine recent advances in our structural understanding of various Gram-negative pilus systems and discuss their functional implications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thanassi, D. G., Bliska, J. B. & Christie, P. J. Surface organelles assembled by secretion systems ofGram-negative bacteria: diversity in structure and function. FEMS Microbiol. Rev. 36, 1046–1082 (2012).
Costa, T. R. D. et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 13, 343–359 (2015).
Fronzes, R., Remaut, H. & Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 27, 2271–2280 (2008).
Arutyunov, D. & Frost, L. S. F conjugation: back to the beginning. Plasmid 70, 18–32 (2013).
Lillington, J., Geibel, S. & Waksman, G. Reprint of 'Biogenesis and adhesion of type 1 and P pili'. Biochim. Biophys. Acta 1850, 554–564 (2015).
Berry, J.-L. & Pelicic, V. Exceptionally widespread nanomachines composed of type IV pilins: the prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 39, 134–154 (2015).
Van Gerven, N., Klein, R. D., Hultgren, S. J. & Remaut, H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 23, 693–706 (2015).
Thanassi, D. G., Saulino, E. T. & Hultgren, S. J. The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr. Opin. Microbiol. 1, 223–231 (1998).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
McLellan, L. K. & Hunstad, D. A. Urinary tract infection: pathogenesis and outlook. Trends Mol. Med. 22, 946–957 (2016).
Schaeffer, A. J., Schwan, W. R., Hultgren, S. J. & Duncan, J. L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55, 373–380 (1987).
Schwan, W. R. Regulation of fim genes in uropathogenic Escherichia coli. World J. Clin. Infect. Dis. 1, 17–25 (2011).
Sauer, F. G., Remaut, H., Hultgren, S. J. & Waksman, G. Fiber assembly by the chaperone–usher pathway. Biochim. Biophys. Acta 1694, 259–267 (2004).
Choudhury, D. et al. X-Ray structure of the FimC–FimH chaperone–adhesin complex from uropathogenic Escherichia coli. Science 285, 1061–1066 (1999).
Dodson, K. W. et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105, 733–743 (2001).
Stathopoulos, C. et al. Secretion of virulence determinants by the general secretory pathway in Gram-negative pathogens: an evolving story. Microbes Infect. 2, 1061–1072 (2000).
Sauer, F. G. et al. Structural basis of chaperone function and pilus biogenesis. Science 285, 1058–1061 (1999).
Barnhart, M. M. et al. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl Acad. Sci. USA 97, 7709–7714 (2000).
Vetsch, M. et al. Pilus chaperones represent a new type of protein-folding catalyst. Nature 431, 329–332 (2004).
Sauer, F. G., Pinkner, J. S., Waksman, G. & Hultgren, S. J. Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell 111, 543–551 (2002).
Zavialov, A. V. et al. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell 113, 587–596 (2003).
Nishiyama, M., Ishikawa, T., Rechsteiner, H. & Glockshuber, R. Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science 320, 376–379 (2008).
Waksman, G. & Hultgren, S. J. Structural biology of the chaperone–usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 7, 765–774 (2009).
Remaut, H. et al. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133, 640–652 (2008).
Huang, Y., Smith, B. S., Chen, L. X., Baxter, R. H. G. & Deisenhofer, J. Insights into pilus assembly and secretion from the structure and functional characterization of usher PapC. Proc. Natl Acad. Sci. USA 106, 7403–7407 (2009).
Ng, T. W., Akman, L., Osisami, M. & Thanassi, D. G. The usher N terminus is the initial targeting site for chaperone-subunit complexes and participates in subsequent pilus biogenesis events. J. Bacteriol. 186, 5321–5331 (2004).
Nishiyama, M., Vetsch, M., Puorger, C., Jelesarov, I. & Glockshuber, R. Identification and characterization of the chaperone–subunit complex-binding domain from the type 1 pilus assembly platform FimD. J. Mol. Biol. 330, 513–525 (2003).
Henderson, N. S., Ng, T. W., Talukder, I. & Thanassi, D. G. Function of the usher N-terminus in catalysing pilus assembly. Mol. Microbiol. 79, 954–967 (2010).
Nishiyama, M. et al. Structural basis of chaperone–subunit complex recognition by the type 1 pilus assembly platform FimD. EMBO J. 24, 2075–2086 (2005).
Phan, G. et al. Crystal structure of the FimD usher bound to its cognate FimC–FimH substrate. Nature 474, 49–53 (2011). This article describes the crystal structure of the FimD usher bound to a complex of FimC (chaperone) and FimH (adhesin), with the FimH lectin domain traversing through the usher pore.
Remaut, H. et al. Donor-strand exchange in chaperone-assisted pilus assembly proceeds through a concerted beta strand displacement mechanism. Mol. Cell 22, 831–842 (2006).
Rose, R. J. et al. Donor-strand exchange in chaperone-assisted pilus assembly revealed in atomic detail by molecular dynamics. J. Mol. Biol. 375, 908–919 (2008).
Werneburg, G. T. et al. The pilus usher controls protein interactions via domain masking and is functional as an oligomer. Nat. Struct. Mol. Biol. 22, 540–546 (2015).
Geibel, S., Procko, E., Hultgren, S. J., Baker, D. & Waksman, G. Structural and energetic basis of folded-protein transport by the FimD usher. Nature 496, 243–246 (2013). This article describes the crystal structure of the FimD usher in the process of translocating the entire type 1 tip fibrillum (FimF–FimG–FimH), and reveals the conformational changes that are required to prevent backsliding of the nascent pilus through the usher.
Verger, D., Miller, E., Remaut, H., Waksman, G. & Hultgren, S. Molecular mechanism of P pilus termination in uropathogenic Escherichia coli. EMBO Rep. 7, 1228–1232 (2006).
Le Trong, I. et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like sheet twisting. Cell 141, 645–655 (2010).
Hannan, T. J. et al. Host–pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 36, 616–648 (2012).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Roberts, J. A. et al. The Gal(α1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA 91, 11889–11893 (1994).
Spaulding, C. & Hultgren, S. Adhesive pili in UTI pathogenesis and drug development. Pathogens 5, E30 (2016).
Thomas, W. E., Trintchina, E., Forero, M., Vogel, V. & Sokurenko, E. V. Bacterial adhesion to target cells enhanced by shear force. Cell 109, 913–923 (2002).
Aprikian, P. et al. Interdomain interaction in the FimH adhesin of Escherichia coli regulates the affinity to mannose. J. Biol. Chem. 282, 23437–23446 (2007).
Yakovenko, O. et al. FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation. J. Biol. Chem. 283, 11596–11605 (2008).
Sauer, M. M. et al. Catch-bond mechanism of the bacterial adhesin FimH. Nat. Commun. 7, 1–13 (2016).
Habenstein, B. et al. Hybrid structure of the type 1 pilus of uropathogenic Escherichia coli. Angew. Chem. Int. Ed. 127, 11857–11861 (2015).
Hospenthal, M. K. et al. Structure of a chaperone–usher pilus reveals the molecular basis of rod uncoiling. Cell 164, 269–278 (2016). This study describes the atomic model of the P pilus derived from a ∼3.8 Å cryo-EM map and reveals the interaction network that forms the quaternary superhelical chaperone–usher pilus structure. This is important for understanding how chaperone–usher pili can reversibly uncoil in response to flow-induced forces.
Puorger, C. et al. Infinite kinetic stability against dissociation of supramolecular protein complexes through donor strand complementation. Structure 16, 631–642 (2008).
Jass, J. et al. Physical properties of Escherichia coli P pili measured by optical tweezers. Biophys. J. 87, 4271–4283 (2004).
Fällman, E., Schedin, S., Jass, J., Uhlin, B. E. & Axner, O. The unfolding of the P pili quaternary structure by stretching is reversible, not plastic. EMBO Rep. 6, 52–56 (2005).
Andersson, M., Fällman, E., Uhlin, B. E. & Axner, O. Dynamic force spectroscopy of E. coli P pili. Biophys. J. 91, 2717–2725 (2006).
Zakrisson, J., Wiklund, K., Axner, O. & Andersson, M. The shaft of the type 1 fimbriae regulates an external force to match the FimH catch bond. Biophys. J. 104, 2137–2148 (2013).
Wülfing, C. & Plückthun, A. Protein folding in the periplasm of Escherichia coli. Mol. Microbiol. 12, 685–692 (1994).
Hahn, H. P. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa — a review. Gene 192, 99–108 (1997).
Pohlschroder, M., Ghosh, A., Tripeti, M. & Albers, S. V. Archaeal type IV pilus-like structures — evolutionarily conserved prokaryotic surface organelles. Curr. Opin. Microbiol. 14, 347–353 (2011).
Melville, S. & Craig, L. Type IV pili in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 77, 323–341 (2013).
Albers, S. V. & Jarrell, K. F. The archaellum: how Archaea swim. Front. Microbiol. 6, 1–12 (2015).
Malvankar, N. S. & Lovley, D. R. Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem 5, 1039–1046 (2012).
Mattick, J. S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Burrows, L. L. Weapons of mass retraction. Mol. Microbiol. 57, 878–888 (2005).
Maier, B., Potter, L., So, M., Seifert, H. S. & Sheetz, M. P. Single pilus motor forces exceed 100 pN. Proc. Natl Acad. Sci. USA 99, 16012–16017 (2002).
Pelicic, V. Type IV pili: e pluribus unum? Mol. Microbiol. 68, 827–837 (2008).
Lory, S. & Strom, M. S. Structure–function relationship of type-IV prepilin peptidase of Pseudomonas aeruginosa — a review. Gene 192, 117–121 (1997).
Zhang, H. Z., Lory, S. & Donnenberg, M. S. A. Plasmid-encoded prepilin pepdidase gene from enteropathogenic Escherichia coli. J. Bacteriol. 176, 6885–6891 (1994).
Leighton, T. L., Buensuceso, R. N. C., Howell, P. L. & Burrows, L. L. Biogenesis of Pseudomonas aeruginosa type IV pili and regulation of their function. Environ. Microbiol. 17, 4148–4163 (2015).
Collins, R. F. et al. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J. Biol. Chem. 279, 39750–39756 (2004).
Burkhardt, J., Vonck, J. & Averhoff, B. Structure and function of PilQ, a secretin of the DNA transporter from the thermophilic bacterium Thermus thermophilus HB27. J. Biol. Chem. 286, 9977–9984 (2011).
Koo, J., Lamers, R. P., Rubinstein, J. L., Burrows, L. L. & Howell, P. L. Structure of the Pseudomonas aeruginosa type IVa pilus secretin at 7.4 Å. Structure 24, 1778–1787 (2016). This work describes the ∼7.4 Å cryo-EM structure of the P. aeruginosa T4P secretin and shows that it is a homo 14-mer that has C7 symmetry.
Korotkov, K. V., Gonen, T. & Hol, W. G. J. Secretins: dynamic channels for protein transport across membranes. Trends Biochem. Sci. 36, 433–443 (2011).
Koo, J. et al. PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa Type IV pilus secretin. J. Bacteriol. 190, 6961–6969 (2008).
Siewering, K. et al. Peptidoglycan-binding protein TsaP functions in surface assembly of type IV pili. Proc. Natl Acad. Sci. USA 111, E953–E961 (2014).
Chiang, P., Habash, M. & Burrows, L. L. Disparate subcellular localization patterns of Pseudomonas aeruginosa type IV pilus ATPases involved in twitching motility. J. Bacteriol. 187, 829–839 (2005).
Takhar, H. K., Kemp, K., Kim, M., Howell, P. L. & Burrows, L. L. The platform protein is essential for type IV pilus biogenesis. J. Biol. Chem. 288, 9721–9728 (2013).
Sandkvist, M., Bagdasarian, M., Howard, S. P. & DiRita, V. J. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 14, 1664–1673 (1995).
Ayers, M. et al. PilM/N/O/P proteins form an inner membrane complex that affects the stability of the Pseudomonas aeruginosa type IV pilus secretin. J. Mol. Biol. 394, 128–142 (2009).
Tammam, S. et al. PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. J. Bacteriol. 195, 2126–2135 (2013).
Sampaleanu, L. M. et al. Periplasmic domains of Pseudomonas aeruginosa PilN and PilO form a stable heterodimeric complex. J. Mol. Biol. 394, 143–159 (2009).
Karuppiah, V. & Derrick, J. P. Structure of the PilM–PilN inner membrane type IV pilus biogenesis complex from Thermus thermophilus. J. Biol. Chem. 286, 24434–24442 (2011).
Georgiadou, M., Castagnini, M., Karimova, G., Ladant, D. & Pelicic, V. Large-scale study of the interactions between proteins involved in type IV pilus biology in Neisseria meningitidis: characterization of a subcomplex involved in pilus assembly. Mol. Microbiol. 84, 857–873 (2012).
Leighton, T. L., Dayalani, N., Sampaleanu, L. M., Howell, P. L. & Burrows, L. L. Novel role for PilNO in type IV pilus retraction revealed by alignment subcomplex mutations. J. Bacteriol. 197, 2229–2238 (2015).
Berry, J.-L. et al. Structure and assembly of a trans-periplasmic channel for type IV pili in Neisseria meningitidis. PLoS Pathog. 8, e1002923 (2012).
Craig, L., Pique, M. E. & Tainer, J. A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2, 363–378 (2004).
Nguyen, Y. et al. Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin. J. Biol. Chem. 290, 601–611 (2015).
Giltner, C. L., Nguyen, Y. & Burrows, L. L. Type IV pilin proteins: versatile molecular modules. Microbiol. Mol. Biol. Rev. 76, 740–772 (2012).
Gold, V. A. M., Salzer, R., Averhoff, B. & Kühlbrandt, W. Structure of a type IV pilus machinery in the open and closed state. eLife 4, 1–12 (2015). This work describes a cryo-ET study of the T4P system in T. thermophilus. The fully assembled T4P system is studied in the non-piliated (closed) and piliated (open) states.
Chang, Y.-W. et al. Architecture of the type IVa pilus machine. Science 351, aad2001 (2016). This article studies the T4P apparatus of M. xanthus using cryo-ET. The non-piliated (closed) and piliated (open) states are investigated and the individual protein components are localized in the cryo-ET map. The authors build an architectural model of the T4P system by fitting existing structures into their cryo-ET map.
Castán, P. et al. The periplasmic space in Thermus thermophilus: evidence from a regulation-defective S-layer mutant overexpressing an alkaline phosphatase. Extremophiles 6, 225–232 (2002).
Quintela, J. C., Pittenauer, E., Allmaier, G., Arán, V. & de Pedro, M. Structure of peptidoglycan from Thermus thermophilus HB8. J. Bacteriol. 177, 4947–4962 (1995).
Worrall, L. J. et al. Near-atomic-resolution cryo-EM analysis of the Salmonella T3S injectisome basal body. Nature 540, 597–601 (2016).
Yan, Z., Yin, M., Xu, D., Zhu, Y. & Li, X. Structural insights into the secretin translocation channel in the type II secretion system. Nat. Struct. Mol. Biol. 24, 177–183 (2017).
Diepold, A., Kudryashev, M., Delalez, N. J., Berry, R. M. & Armitage, J. P. Composition, formation, and regulation of the cytosolic C-ring, a dynamic component of the type III secretion injectisome. PLoS Biol. 13, e1002039 (2015).
Craig, L. et al. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23, 651–662 (2006).
Kolappan, S. et al. Structure of the Neisseria meningitidis type IV pilus. Nat. Commun. 7, 1–12 (2016). In this article, the atomic model of the N. meningitidis T4aP is built by fitting a 1.44 Å pilin crystal structure (PilE; a major pilin in N. meningitidis ) into a ∼6 Å cryo-EM volume. This reveals how the N-terminal α-helical regions of PilE pack together in the core of the structure and describes a model of how T4P could stretch in response to tensile forces.
Parge, H. E. et al. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 378, 32–38 (1995).
Craig, L. et al. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11, 1139–1150 (2003).
Bischof, L. F., Friedrich, C., Harms, A., Søgaard-Andersen, L. & van der Does, C. The type IV pilus assembly ATPase PilB of Myxococcus xanthus interacts with the inner membrane platform protein PilC and the nucleotide-binding protein PilM. J. Biol. Chem. 291, 6946–6957 (2016).
Mancl, J. M., Black, W. P., Robinson, H., Yang, Z. & Schubot, F. D. Crystal structure of a type IV pilus assembly ATPase: insights into the molecular mechanism of PilB from Thermus thermophilus. Structure 24, 1886–1897 (2016).
Thomas, C. M. & Nielsen, K. M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3, 711–721 (2005).
Ilangovan, A., Connery, S. & Waksman, G. Structural biology of the Gram-negative bacterial conjugation systems. Trends Microbiol. 23, 301–310 (2015).
Daehnel, K. Fluorescence assays for F-pili and their application. Microbiology 151, 3541–3548 (2005).
Guglielmini, J., de la Cruz, F. & Rocha, E. P. C. Evolution of conjugation and type IV secretion systems. Mol. Biol. Evol. 30, 315–331 (2013).
Garcillán-Barcia, M. P., Alvarado, A. & de la Cruz, F. Identification of bacterial plasmids based on mobility and plasmid population biology. FEMS Microbiol. Rev. 35, 936–956 (2011).
Costa, T. R. D. et al. Structure of the bacterial sex F pilus reveals an assembly of a stoichiometric protein–phospholipid complex. Cell 166, 1436–1444 (2016). This study provides the first structural insight into a conjugative pilus determined by cryo-EM at a resolution of ∼3.6 Å, which reveals a pilus built by a protein–phospholipid complex in a stoichiometric 1:1 ratio.
Majdalani, N., Moore, D., Maneewannakul, S. & Ippen-Ihler, K. Role of the propilin leader peptide in the maturation of F pilin. J. Bacteriol. 178, 3748–3754 (1996).
Majdalani, N. & Ippen-Ihler, K. Membrane insertion of the F-pilin subunit is Sec independent but requires leader peptidase B and the proton motive force. J. Bacteriol. 178, 3742–3747 (1996).
Maneewannakul, K., Maneewannakul, S. & Ippen-Ihler, K. Characterization of traX, the F plasmid locus required for acetylation of F-pilin subunits. J. Bacteriol. 177, 2957–2964 (1995).
Chandran Darbari, V. & Waksman, G. Structural biology of bacterial type IV secretion systems. Annu. Rev. Biochem. 84, 603–629 (2015).
Low, H. H. et al. Structure of a type IV secretion system. Nature 508, 550–553 (2014). This study is the first to unveil the overall architecture of a type IV secretion system.
Fronzes, R. et al. Structure of a type IV secretion system core complex. Science 323, 266–268 (2009). This paper describes the cryo-EM structure of the T4SS OMC that inserts in both the outer membrane and the inner membrane of Gram-negative bacteria.
Rivera-Calzada, A. et al. Structure of a bacterial type IV secretion core complex at subnanometre resolution. EMBO J. 32, 1195–1204 (2013).
Chandran, V. et al. Structure of the outer membrane complex of a type IV secretion system. Nature 462, 1011–1015 (2009). This article describes the crystal structure of an approximately 0.6 MDa OMC that contains the entire O-layer.
Ghosal, D., Chang, Y.-W., Jeong, K. C., Vogel, J. P. & Jensen, G. J. In situ structure of the Legionella Dot/Icm type IV secretion system by electron cryotomography. EMBO Rep. http://dx.doi.org/10.15252/embr.201643598 (2017).
Kerr, J. E. & Christie, P. J. Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system. J. Bacteriol. 192, 4923–4934 (2010).
Ripoll-Rozada, J., Zunzunegui, S., de la Cruz, F., Arechaga, I. & Cabezón, E. Functional interactions of VirB11 traffic ATPases with VirB4 and VirD4 molecular motors in type IV secretion systems. J. Bacteriol. 195, 4195–4201 (2013).
Cao, T. B. & Saier, M. H. Conjugal type IV macromolecular transfer systems of Gram-negative bacteria: organismal distribution, structural constraints and evolutionary conclusions. Microbiology 147, 3201–3214 (2001).
Holt, S. C. & Ebersole, J. L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the 'red complex', a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 38, 72–122 (2000).
Yoshimura, F., Murakami, Y., Nishikawa, K., Hasegawa, Y. & Kawaminami, S. Surface components of Porphyromonas gingivalis. J. Periodontal Res. 44, 1–12 (2009).
Xu, Q. et al. A distinct type of pilus from the human microbiome. Cell 165, 690–703 (2016). This article reveals 20 crystal structures of type V pilins, including tip, stalk and anchor pilins, and highlights their structural differences that are important for the assembly and function of type V pili. This work also describes the C-terminal strand-exchange mechanism that is important for the assembly of type V pili.
Yoshimura, F., Takahashi, K., Nodosaka, Y. & Suzuki, T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J. Bacteriol. 160, 949–957 (1984).
Hamada, N., Sojar, H. T., Cho, M. I. & Genco, R. J. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect. Immun. 64, 4788–4794 (1996).
Kloppsteck, P., Hall, M., Hasegawa, Y. & Persson, K. Structure of the fimbrial protein Mfa4 from Porphyromonas gingivalis in its precursor form: implications for a donor-strand complementation mechanism. Sci. Rep. 6, 22945 (2016). This article reveals the 1.9 Å crystal structure of Mfa4 (a type V pilin) and proposes a strand-exchange mechanism for type V pilus assembly, whereby an N-terminal strand complements a groove of the neighbouring subunit during assembly.
Xu, Q. et al. A conserved fold for fimbrial components revealed by the crystal structure of a putative fimbrial assembly protein (BT1062) from Bacteroides thetaiotaomicron at 2.2 Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1281–1286 (2010).
Shoji, M. et al. The major structural components of two cell surface filaments of Porphyromonas gingivalis are matured through lipoprotein precursors. Mol. Microbiol. 52, 1513–1525 (2004).
Nakayama, K., Yoshimura, F., Kadowaki, T. & Yamamoto, K. Involvement of arginine-specific cysteine proteinase (Arg-gingipain) in fimbriation of Porphyromonas gingivalis. J. Bacteriol. 178, 2818–2824 (1996).
Chapman, M. R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Cao, B. et al. Structure of the nonameric bacterial amyloid secretion channel. Proc. Natl Acad. Sci. USA 111, E5439–E5444 (2014). Together with reference 126, this study describes the atomic details of the outer membrane secretion channel that is formed by the CsgG lipoprotein. The channel provides insights into how the secretion of curli subunits occurs in an unfolded state.
Goyal, P. et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 516, 250–253 (2014).
Nenninger, A. A. et al. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol. Microbiol. 81, 486–499 (2011).
Hammer, N. D. et al. The C-terminal repeating units of CsgB direct bacterial functional amyloid nucleation. J. Mol. Biol. 422, 376–389 (2012).
Nenninger, A. A., Robinson, L. S. & Hultgren, S. J. Localized and efficient curli nucleation requires the chaperone-like amyloid assembly protein CsgF. Proc. Natl Acad. Sci. USA 106, 900–905 (2009).
Eidam, O., Dworkowski, F. S. N., Glockshuber, R., Grütter, M. G. & Capitani, G. Crystal structure of the ternary FimC–FimFt–FimDN complex indicates conserved pilus chaperone-subunit complex recognition by the usher FimD. FEBS Lett. 582, 651–655 (2008).
Acknowledgements
This work was funded by the UK Medical Research Council (MRC; grant 018434) and the Wellcome Trust (grant 098302 to G.W.). The authors apologize for any omissions owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Pili
-
Long non-flagellar appendages at the cell surface, also referred to as fimbriae, that are present in a wide range of Gram-negative and Gram-positive bacteria and in archaea, and are involved in bacterial attachment, motility and horizontal gene transfer.
- Biofilm
-
A community of bacterial cells that form a dense surface-associated matrix of proteins, nucleic acids and polysaccharides that provides a strong fitness advantage, such as an enhanced tolerance to antibiotics and a reduced susceptibility to host immune responses and other physical and chemical stresses.
- Type IV pili
-
(T4P). Widespread surface appendages and important virulence factors that are used by bacteria to enable attachment, biofilm formation and both twitching and gliding motility.
- Pilins
-
Pilus subunits, which form a pilus in their assembled state. A pilus can contain several thousand copies of a single pilin or may be composed of more than one type of pilin. In some pili, the terms 'tip pilin' and 'anchor pilin' refer to pilins that are present at the tip or the base of the pilus structure, respectively.
- Lectin domain
-
A versatile carbohydrate-binding domain found in many Gram-negative bacterial pili that enables bacteria to attach to host tissues during infection.
- SecYEG translocon
-
An evolutionarily conserved membrane transporter that is located in the cytoplasmic membrane of bacteria and archaea, and the membrane of the endoplasmic reticulum in eukaryotic cells. In bacteria, this machinery transports proteins into the periplasm and can insert membrane proteins into the inner membrane.
- Donor-strand complementation
-
(DSC). A mechanism whereby an incomplete immunoglobulin-like fold in a pilus subunit is completed and stabilized in the periplasm by a donor strand from a dedicated periplasmic chaperone (either FimC or PapD).
- Usher
-
An outer membrane-embedded protein that catalyses the assembly of chaperone–usher pili. This protein is composed of a 24-stranded β-barrel pore domain, a periplasmic amino-terminal domain (NTD), two periplasmic carboxy-terminal domains (CTD1 and CTD2) and a plug domain.
- Donor-strand exchange
-
(DSE). A mechanism whereby an incomplete immunoglobulin-like fold in a pilus subunit is completed and stabilized by a donor strand that is provided by the amino-terminal extension of an adjacent pilus subunit. This occurs once pilus subunits are assembled into the growing pilus by the usher.
- Type II secretion systems
-
(T2SSs). Large macromolecular nanomachines present in various pathogenic and non-pathogenic Gram-negative bacteria that are responsible for the secretion of folded proteins (including enzymes and toxins) from the periplasm to the extracellular environment.
- Secretin
-
Large, multimeric and gated outer membrane pore-forming proteins that are found in type IV pilus systems, type II and type III secretion systems, and in some filamentous bacteriophage extrusion systems.
- Pilotin
-
Proteins that function to ensure correct secretin localization, assembly and outer membrane insertion.
- Amidase N-terminal domains
-
(AMIN domains). Domains that are widely distributed among bacterial peptidoglycan hydrolases and transporters located in the periplasm, and are thought to interact with the peptidoglycan cell wall. The presence of AMIN domains in secretins is thought to help anchor the type IV pilus machinery in the cell wall.
- Conjugation
-
A mechanism of horizontal gene transfer that involves the transfer of genetic material from a donor to a recipient bacterial cell.
- Donor cell
-
A cell that provides a conjugative genetic element, which is often a plasmid or an integrative conjugative element, that is eventually mobilized to a recipient cell at some point in the bacterial life cycle.
- Integrative conjugative elements
-
(ICE). A large family of chromosomally encoded mobile genetic elements that encode a functional conjugative secretion system that mediates their excision, transfer and integration into a recipient cell. Integrative conjugative elements are also known as conjugative transposons.
- Recipient cell
-
A cell that acquires a conjugative genetic element from a donor cell, which either gets incorporated into the bacterial chromosome (for example, integrative conjugative elements) or remains in the bacterial cytoplasm (for example, a plasmid).
- Type IV secretion system
-
(T4SS). A large macromolecular nanomachine that is found in both Gram-positive and Gram-negative bacteria, as well in some archaea. These systems have evolved to deliver DNA and protein substrates into a wide range of prokaryotic and eukaryotic target cells, which promotes the spread of antibiotic resistance and bacterial pathogenesis.
- Proton motive force
-
(PMF). An electrochemical gradient across a bacterial cell membrane that is generated by the transfer of protons or electrons across an energy-transducing membrane by an electron transport chain.
Rights and permissions
About this article
Cite this article
Hospenthal, M., Costa, T. & Waksman, G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat Rev Microbiol 15, 365–379 (2017). https://doi.org/10.1038/nrmicro.2017.40
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro.2017.40
This article is cited by
-
High-level polymyxin B resistance and underlying mechanism in a multidrug-resistant Acinetobacter strain isolated from the marine plastisphere
The Journal of Antibiotics (2026)
-
Tad pili with adaptable tips mediate contact-dependent killing during bacterial predation
Nature Communications (2025)
-
Bacterial adhesion strategies and countermeasures in urinary tract infection
Nature Microbiology (2025)
-
Structures of the Escherichia coli type 1 pilus during pilus rod assembly and after assembly termination
Nature Communications (2025)
-
Type IV secretion systems: from structures to mechanisms
The EMBO Journal (2025)