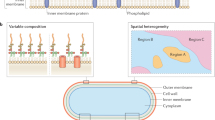
Key Points
-
Bacterial mechanosensitive (MS) channels are gated by the perturbation of membrane tension, forming non-selective pores of 16–40 Å through which hydrated ions and solutes can flow.
-
MS channels have a key role in the survival of hypoosmotic shock, but might also have other roles during cell-wall remodelling.
-
Using electrophysiology, three principal structural classes of MS channels have been defined in Escherichia coli. Species can have multiple homologues of each class, but not all have demonstrated MS channel activity. The homologues differ in their degree of conservation of the pore-lining helix residues, and their threshold sensitivity to tension might reflect this and be selected to enable them to have specific cellular functions.
-
MscS and MscL homologues are also found in plants, oomycetes, algae and in some fungi. MscS homologues are associated with chloroplast shape and development in Arabidopsis and Chlamydomonas.
-
Crystal structures of the Mycobacterium tuberculosis MscL (MscL-Mt) and the E. coli MscS (MscS-Ec) channels have revealed a homopentamer and a homoheptamer respectively. Structurally the channels are unrelated, as MscS has more complex packing and an extensive cytoplasmic domain that is required for assembly. Both MscL and MscS use a hydrophobic seal to maintain the channel pore in the closed state.
-
The emerging consensus is that the crystal structures represent either closed states or intermediates in the transition from closed to open states. MscS-Ec characteristically exhibits a desensitized, inactivated state and it is possible that the crystal structure is in this conformation rather than in the 'natural' closed state. MscL-Mt is generally accepted to have been crystallized in the closed state.
-
The structural transitions in MscL and MscS gating have been studied using biophysical, genetic and biochemical approaches. Both channels gate by tilting and rotating the helices surrounding the pore, which involves specific conserved residues.
-
Recent molecular analysis of MS channels has focused on the interaction of the channel-protein residues with surrounding membrane lipids. In this Review we define the absence of specific amino acids at the protein–lipid interface that might block mechanogating as central to MS channel function. We term this 'negative space'.
Abstract
Bacterial mechanosensitive channels are activated by increases in tension in the lipid bilayer of the cytoplasmic membrane, where they transiently create large pores in a controlled manner. Mechanosensitive channel research has benefited from advances in electrophysiology, genomics and molecular genetics as well as from the application of biophysical techniques. Most recently, new analytical methods have been used to complement existing knowledge and generate insights into the molecular interactions that take place between mechanosensitive channel proteins and the surrounding membrane lipids. This article reviews the latest developments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levina, N. et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18, 1730–1737 (1999).
Sukharev, S. I., Martinac, B., Arshavsky, V. Y. & Kung, C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 (1993).
Sukharev, S. I., Sigurdson, W. J., Kung, C. & Sachs, F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540 (1999).
Sukharev, S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83, 290–298 (2002).
Berrier, C., Coulombe, A., Szabo, I., Zoratti, M. & Ghazi, A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur. J. Biochem. 206, 559–565 (1992).
Schleyer, M., Schmid, R. & Bakker, E. P. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli K-12 exposed to hypoosmotic shock. Arch. Microbiol. 160, 424–431 (1993).
Mitchell, P. & Moyle, J. in Bacterial Anatomy (eds. Spooner, E. & Stocker, B.) 150–180 (Cambridge University Press, Cambridge, 1956).
Imhoff, J. F. Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol. Rev. 39, 57–66 (1986).
Booth, I. R., Cairney, J., Sutherland, L. & Higgins, C. F. Enteric bacteria and osmotic-stress — an integrated homeostatic system. J. Appl. Bacteriol. 65, S35–S49 (1988).
Holtje, J.-V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62, 181–203 (1998).
Stokes, N. R. et al. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc. Natl Acad. Sci. USA 100, 15959–15964 (2003).
Arisaka, F., Kanamaru, S., Leiman, P. & Rossmann, M. G. The tail lysozyme complex of bacteriophage T4. Int. J. Biochem. Cell Biol. 35, 16–21 (2003).
Quintela, J. C., dePedro, M. A., Zollner, P., Allmaier, G. & Garcia del Portillo, F. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol. Microbiol. 23, 693–704 (1997).
Booth, I. R. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49, 359–378 (1985).
Britten, R. J. & McClure, F. T. The amino acid pool in Escherichia coli. Microbiol. Mol. Biol. Rev. 26, 292–335 (1962).
Martinac, B., Buehner, M., Delcour, A. H., Adler, J. & Kung, C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl Acad. Sci. USA 84, 2297–2301 (1987).
Sukharev, S. I., Blount, P., Martinac, B., Blattner, F. R. & Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268 (1994).
Berrier, C., Besnard, M., Ajouz, B., Coulombe, A. & Ghazi, A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol. 151, 175–187 (1996).
Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 (1998). MscL from M. tuberculosis , the first bacterial mechanosensitive channel protein to be crystallized, is shown to consist of five identical subunits that each contain two TM spans with short cytoplasmic helices at either end and a β-strand periplasmic loop between TM1 and TM2.
Bass, R. B., Strop, P., Barclay, M. & Rees, D. C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002). Determination of the E. coli MscS crystal structure, indicating the greater complexity of this protein over that of MscL. MscS contains seven subunits (each with three TM spans and a large multidomain cytoplasmic sequence), which form a homoheptamer.
Kung, C. A possible unifying principle for mechanosensation. Nature 436, 647–654 (2005).
Cruickshank, C. C., Minchin, R. F., Le Dain, A. C. & Martinac, B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73, 1925–1931 (1997).
Li, Y., Moe, P. C., Chandrasekaran, S., Booth, I. R. & Blount, P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21, 5323–5330 (2002). This work investigates MscK, a member of the MscS family, and demonstrates how this channel is regulated by the external K+ concentration, as well as by membrane tension. However, it also shows that the requirement for K+ is eliminated in the presence of a strong gain-of-function mutation.
Ajouz, B., Berrier, C., Garrigues, A., Besnard, M. & Ghazi, A. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J. Biol. Chem. 273, 26670–26674 (1998).
Berrier, C., Garrigues, A., Richarme, G. & Ghazi, A. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel MscL. J. Bacteriol. 182, 248–251 (2000).
Ewis, H. E. & Lu, C. D. Osmotic shock: a mechanosensitive channel blocker can prevent release of cytoplasmic but not periplasmic proteins. FEMS Microbiol. Lett. 253, 295–301 (2005).
Vazquez-Laslop, N., Lee, H., Hu, R. & Neyfakh, A. A. Molecular sieve mechanism of selective release of cytoplasmic proteins by osmotically shocked Escherichia coli. J. Bacteriol. 183, 2399–2404 (2001).
van den Bogaart, G., Krasnikov, V. & Poolman, B. Dual-color fluorescence-burst analysis to probe protein efflux through the mechanosensitive channel MscL. Biophys. J. 92, 1233–1240 (2007).
Folgering, J. H., Kuiper, J. M., de Vries, A. H., Engberts, J. B. & Poolman, B. Lipid-mediated light activation of a mechanosensitive channel of large conductance. Langmuir 20, 6985–6987 (2004).
Booth, I. R. in Genetic Engineering — Principles and Methods (ed. Setlow, J. K.) 91–112 (Kluwer Academic/Plenum Publishers, New York, 2003).
Li, Y., Wray, R. & Blount, P. Intragenic suppression of gain-of-function mutations in the Escherichia coli mechanosensitive channel, MscL. Mol. Microbiol. 53, 485–495 (2004).
Folgering, J. H., Moe, P. C., Schuurman-Wolters, G. K., Blount, P. & Poolman, B. Lactococcus lactis uses MscL as its principal mechanosensitive channel. J. Biol. Chem. 280, 8784–8792 (2005).
Batiza, A. F., Kuo, M. M. C., Yoshimura, K. & Kung, C. Gating the bacterial mechanosensitive channel MscL in vivo. Proc. Natl Acad. Sci. USA 99, 5643–5648 (2002).
Miller, S., Edwards, M. D., Ozdemir, C. & Booth, I. R. The closed structure of the MscS mechanosensitive channel — cross-linking of single cysteine mutants. J. Biol. Chem. 278, 32246–32250 (2003). Crosslinking of single Cys mutants of MscS revealed, for the first time, how the protein takes on a more compact conformational state than that observed in the crystal structure.
Blount, P., Sukharev, S. I., Schroeder, M. J., Nagle, S. K. & Kung, C. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl Acad. Sci. USA 93, 11652–11657 (1996).
Powl, A. M., East, J. M. & Lee, A. G. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry 44, 5873–5883 (2005). Part of a series of work (Refs 65,80 ) investigating the interactions between the MscL protein and the surrounding membrane lipids. This particular study highlights putative lipid-binding sites that have a cluster of three positively charged amino acids on the cytoplasmic side of the membrane.
Schumann, U., Edwards, M. D., Li, C. & Booth, I. R. The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 572, 233–237 (2004).
Miller, S. et al. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22, 36–46 (2003).
Norman, C. et al. Visualisation of the mechanosensitive channel of large conductance in bacteria using confocal microscopy. Eur. Biophys. J. 34, 396–402 (2005).
Anishkin, A. & Sukharev, S. Water dynamics and dewetting transition in the small mechanosensitive channel MscS. Biophys. J. 86, 2883–2895 (2004). Although the original interpretation of the MscS-Ec crystal structure proposed an open state, this paper assesses the properties of the MscS pore residues and uses molecular dynamics simulations to suggest that the crystal structure conformation might not be continuously hydrated and therefore represents a closed, or at least a non-conducting, form of the channel.
Koprowski, P. & Kubalski, A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J. Biol. Chem. 278, 11237–11245 (2003).
Bartlett, J. L., Li, Y. & Blount, P. Mechanosensitive channel gating transitions resolved by functional changes upon pore modification. Biophys. J. 91, 3684–3691 (2006).
Beckstein, O. & Sansom, M. S. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys. Biol. 1, 42–52 (2004). Molecular dynamics is used to analyse the properties required for the passage of ions through a channel lumen. The hydrophobicity and hydrophilicity of the pore-wall residues influences access to water, which in turn influences the ability of ions to pass through the channel. Flow, therefore, is not determined by ion size alone.
Beckstein, O. & Sansom, M. S. Liquid-vapor oscillations of water in hydrophobic nanopores. Proc. Natl Acad. Sci. USA 100, 7063–7068 (2003).
Edwards, M. D. et al. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nature Struct. Mol. Biol. 12, 113–119 (2005). This paper characterizes a number of mutations that disrupt the conserved Ala–Gly packing arrangement seen between MscS pore-lining helices. The data support a model in which these helices rotate and tilt during gating and depend on a knob versus groove mechanism for residues at the packing interface.
Filatov, G. & White, M. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol. Pharmacol. 48, 379–384 (1995).
Ou, X., Blount, P., Hoffman, R. J. & Kung, C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl Acad. Sci. USA 95, 11471–11475 (1998).
Steinbacher, S., Bass, R., Strop, P. & Rees, D. C. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Curr. Top. Membr. 58, 1–24 (2007).
Vora, T., Corry, B. & Chung, S. H. Brownian dynamics investigation into the conductance state of the MscS channel crystal structure. Biochim. Biophys. Acta. 1758, 730–737 (2006).
Kim, S., Chamberlain, A. K. & Bowie, J. U. Membrane channel structure of Helicobacter pylori vacuolating toxin: Role of multiple GXXXG motifs in cylindrical channels. Proc. Natl Acad. Sci. USA 101, 5988–5991 (2004).
Sotomayor, M. & Schulten, K. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys. J. 87, 3050–3065 (2004).
Koprowski, P. & Kubalski, A. Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J. Membr. Biol. 164, 253–262 (1998).
Akitake, B., Anishkin, A. & Sukharev, S. The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 125, 143–154 (2005).
Shapovalov, G. & Lester, H. A. Gating transitions in bacterial ion channels measured at 3 μs resolution. J. Gen. Physiol. 124, 151–161 (2004).
Anishkin, A., Chiang, C. S. & Sukharev, S. Gain-of-function mutations reveal expanded intermediate states and a sequential action of two gates in MscL. J. Gen. Physiol. 125, 155–170 (2005).
Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A. & Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 (2002). An analysis of bilayer mechanical properties — hydrophobic mismatch and membrane curvature — and how they influence the opening of MscL channels.
Perozo, E., Kloda, A., Cortes, D. M. & Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Struct. Biol. 9, 696–703 (2002).
Blount, P., Schroeder, M. J. & Kung, C. Mutations in a bacterial mechanosensitive channel change the cellular response to osmotic stress. J. Biol. Chem. 272, 32150–32157 (1997).
Iscla, I., Levin, G., Wray, R., Reynolds, R. & Blount, P. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys. J. 87, 3172–3180 (2004).
Chiang, C. S., Shirinian, L. & Sukharev, S. Capping transmembrane helices of MscL with aromatic residues changes channel response to membrane stretch. Biochemistry 44, 12589–12597 (2005).
Chiang, C. S., Anishkin, A. & Sukharev, S. Gating of the large mechanosensitive channel in situ: estimation of the spatial scale of the transition from channel population responses. Biophys. J. 86, 2846–2861 (2004).
Sukharev, S., Durell, S. R. & Guy, H. R. Structural models of the MscL gating mechanism. Biophys. J. 81, 917–936 (2001).
Sukharev, S., Betanzos, M., Chiang, C. S. & Guy, H. R. The gating mechanism of the large mechanosensitive channel MscL. Nature 409, 720–724 (2001).
Park, K. H., Berrier, C., Martinac, B. & Ghazi, A. Purification and functional reconstitution of N- and C-halves of the MscL channel. Biophys. J. 86, 2129–2136 (2004).
Powl, A. M., East, J. M. & Lee, A. G. Lipid–protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry 42, 14306–14317 (2003).
Martinac, B., Adler, J. & Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348, 261–263 (1990).
Nomura, T., Sokabe, M. & Yoshimura, K. Lipid-protein interaction of the MscS mechanosensitive channel examined by scanning mutagenesis. Biophys. J. 91, 2874–2881 (2006).
Yoshimura, K., Nomura, T. & Sokabe, M. Loss-of-function mutations at the rim of the funnel of mechanosensitive channel MscL. Biophys. J. 86, 2113–2120 (2004). Mutational analysis of MscL residues located towards the periplasmic ends of the TM segments suggests an interaction with lipid headgroups that contributes to the gating mechanism.
von Heijne, G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225, 487–494 (1992).
von Heijne, G. Membrane-protein topology. Nature Rev. Mol. Cell. Biol. 7, 909–918 (2006).
White, S. H. & Wimley, W. C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 (1999).
Jiang, Y. X. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003).
Okada, K., Moe, P. C. & Blount, P. Functional design of bacterial mechanosensitive channels. Comparisons and contrasts illuminated by random mutagenesis. J. Biol. Chem. 277, 27682–27688 (2002).
Palenik, B. et al. Genome sequence of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc. Natl Acad. Sci. USA 103, 13555–13559 (2006).
Haswell, E. S. & Meyerowitz, E. M. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 16, 1–11 (2006).
Nakayama, Y., Fujiu, K., Sokabe, M. & Yoshimura, K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc. Natl Acad. Sci. USA 104, 5883–5888 (2007).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981).
Delcour, A. H., Martinac, B., Adler, J. & Kung, C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 56, 631–636 (1989).
Kloda, A. & Martinac, B. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 20, 1888–1896 (2001).
Powl, A. M., Wright, J. N., East, J. M. & Lee, A. G. Identification of the hydrophobic thickness of a membrane protein using fluorescence spectroscopy: studies with the mechanosensitive channel MscL. Biochemistry 44, 5713–5721 (2005).
Perozo, E., Kloda, A., Cortes, D. M. & Martinac, B. Site-directed spin-labeling analysis of reconstituted MscL in the closed state. J. Gen. Physiol. 118, 193–206 (2001).
Levin, G. & Blount, P. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys. J. 86, 2862–2870 (2004).
Iscla, I., Levin, G., Wray, R. & Blount, P. Disulfide trapping the mechanosensitive channel MscL into a gating-transition state. Biophys. J. 92, 1224–1232 (2007).
Li, C., Edwards, M. D., Hocherl, J., Roth, J. & Booth, I. R. Identification of mutations that alter the gating of the E. coli mechanosensitive channel protein, MscK. Mol. Microbiol. 64, 560–574 (2007).
Buurman, E. T., McLaggan, D., Naprstek, J. & Epstein, W. Multiple paths for aberrant transport of K+ in Escherichia coli. J. Bacteriol. 186, 4238–4245 (2004).
Booth, I. R. et al. in Methods in Enzymology (eds. Sies, M. & Haeussinger, D.)(Elsevier, 2007).
Beckstein, O. & Sansom, M. S. A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Phys. Biol. 3, 147–159 (2006).
Acknowledgements
The authors acknowledge the generous support of their research collaborators and colleagues, but in particular P. Blount, J. Bowie, J. Naismith, T. Rasmussen, A. Rasmussen, W. Bartlett, C. Kung, B. Martinac, D. Rees, E. Perozo, T. Lee and S. Sukharev. Research on MS channels is supported by The Wellcome Trust (GR077564MA), the Biotechnology and Biological Sciences Research Council (BBSRC), MRC and the University of Aberdeen, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Patch clamp
-
A technique whereby a small glass electrode tip is tightly sealed onto a patch of cell membrane, thereby making it possible to record the flow of current through individual ion channels or pores in the patch.
- Conductance
-
Calculated from the increase in current when a single channel is fully open, under known conditions of applied transmembrane voltage.
- Open dwell time
-
The average time a single channel remains in the fully open state under conditions of constant transmembrane pressure and voltage; this parameter can only be determined statistically based on the analysis of many single openings of channels that occur over several minutes in a patch-clamp recording.
- Pressure sensitivity
-
The pressure required to open channels is most often quoted relative to another channel. For example, the pressure required to open MscL-Ec is often quoted as a ratio by reference to the pressure required to achieve the first openings of MscS-Ec in the same membrane patch. Absolute measures of sensitivity to membrane tension can be achieved only by measuring the curvature of the patch under pressure using video microscopy and by applying Laplace's law, which relates the tension in the bilayer to the transmembrane pressure through the radius of the curvature of the patch.
- Protonmotive force
-
The protonmotive force is created when protons are expelled from the cell during respiratory and photosynthetic electron flow or by the action of an ATPase. The protonmotive force consists of the proton gradient (ΔpH) and a gradient of charge (ΔΨ). Proton (and Na+) ions enter the cell, driven by the protonmotive force, to do useful work such as ATP synthesis, flagellar rotation and membrane transport.
- Inactivation
-
(also known as desensitization). MscS-Ec has been observed to undergo spontaneous loss of channel activity when held under constant pressure; activity can be restored to the majority of channels in a patch by resting the membrane (re-setting the pressure to zero) for a short period before re-imposing pressure.
- Electron paramagnetic resonance
-
Observation of the transitions between spin states of an unpaired electron in a magnetic field.
Rights and permissions
About this article
Cite this article
Booth, I., Edwards, M., Black, S. et al. Mechanosensitive channels in bacteria: signs of closure?. Nat Rev Microbiol 5, 431–440 (2007). https://doi.org/10.1038/nrmicro1659
Issue date:
DOI: https://doi.org/10.1038/nrmicro1659
This article is cited by
-
Genome-wide analysis of OSCA gene family members in Vigna radiata and their involvement in the osmotic response
BMC Plant Biology (2021)
-
High-pressure processing-induced transcriptome response during recovery of Listeria monocytogenes
BMC Genomics (2021)
-
ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens
Nature Communications (2019)
-
Large plasmidome of dairy Lactococcus lactis subsp. lactis biovar diacetylactis FM03P encodes technological functions and appears highly unstable
BMC Genomics (2018)
-
On the mobility, membrane location and functionality of mechanosensitive channels in Escherichia coli
Scientific Reports (2016)