Key Points
-
Animal models are essential for testing antiretroviral drugs to treat HIV infection of humans and for acquiring the basic scientific knowledge that will ultimately be needed to develop a safe and effective vaccine against HIV-1.
-
Owing to the narrow host range of HIV, HIV-1 infection of mice that have been reconstituted with a human immune system (humanized mice) and simian immunodeficiency virus (SIV) infection of macaques are used as surrogate models for studying HIV-1 infection of humans.
-
There is no single animal model for AIDS, but rather a collection of host species and viruses that can be used depending on the question to be addressed.
-
A number of humanized mouse models have been developed using different genetic backgrounds and engraftment with various human tissues. Whereas humanized mice make it possible to model HIV-1 infection of human cells in vivo and to design studies using genetically identical animals, these models are limited in their ability to replicate the effects of HIV-1 on non-haematopoietic tissues and to recreate basic features of HIV-1 disease in humans.
-
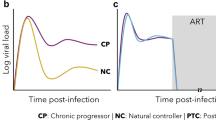
Infection of Asian macaques with certain SIV or simian–human immunodeficiency virus (SHIV) recombinants results in gradual CD4+ T cell depletion and progression to AIDS, similar to HIV infection of humans. Thus, SIV and SHIV infection of macaques are currently the best, most widely accepted models for AIDS research.
-
Genetic differences between macaque species, and in some cases between geographically distinct populations of the same species, can dramatically affect the outcome of SIV and SHIV infections and are important considerations in the use of these models.
-
There is now considerable interest in engineering HIV-1 to overcome the barriers to HIV-1 replication in macaques. These barriers are imposed by the apolipoprotein B-editing catalytic subunit-like 3 (APOBEC3) proteins, by tripartite-motif-containing protein 5 (TRIM5) variants and by tetherin, all of which are HIV restriction factors. The development of minimally modified simian-tropic strains of HIV-1 that can reproducibly cause disease in macaques might eventually allow direct efficacy testing of antiretroviral drugs and vaccines in non-human primates.
Abstract
The AIDS pandemic continues to present us with unique scientific and public health challenges. Although the development of effective antiretroviral therapy has been a major triumph, the emergence of drug resistance requires active management of treatment regimens and the continued development of new antiretroviral drugs. Moreover, despite nearly 30 years of intensive investigation, we still lack the basic scientific knowledge necessary to produce a safe and effective vaccine against HIV-1. Animal models offer obvious advantages in the study of HIV/AIDS, allowing for a more invasive investigation of the disease and for preclinical testing of drugs and vaccines. Advances in humanized mouse models, non-human primate immunogenetics and recombinant challenge viruses have greatly increased the number and sophistication of available mouse and simian models. Understanding the advantages and limitations of each of these models is essential for the design of animal studies to guide the development of vaccines and antiretroviral therapies for the prevention and treatment of HIV-1 infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gao, F. et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441 (1999).
Keele, B. F. et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313, 523–526 (2006).
Keele, B. F. et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460, 515–519 (2009).
Alter, H. J. et al. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science 226, 549–552 (1984).
O'Neil, S. P. et al. Progressive infection in a subset of HIV-1-positive chimpanzees. J. Infect. Dis. 182, 1051–1062 (2000).
Hatziioannou, T. & Bieniasz, P. D. Antiretroviral restriction factors. Curr. Opin. Virol. 1, 526–532 (2011).
Morrow, W. J., Wharton, M., Lau, D. & Levy, J. A. Small animals are not susceptible to human immunodeficiency virus infection. J. Gen. Virol. 68, 2253–2257 (1987).
Browning, J. et al. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl Acad. Sci. USA 94, 14637–14641 (1997).
Dunn, C. S. et al. Human immunodeficiency virus type 1 infection of human CD4-transgenic rabbits. J. Gen. Virol. 76, 1327–1336 (1995).
Keppler, O. T. et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195, 719–736 (2002).
Bieniasz, P. D. & Cullen, B. R. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74, 9868–9877 (2000).
Mariani, R. et al. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74, 3859–3870 (2000).
Tervo, H. M. & Keppler, O. T. High natural permissivity of primary rabbit cells for HIV-1, with a virion infectivity defect in macrophages as the final replication barrier. J. Virol. 84, 12300–12314 (2010).
Leonard, J. M. et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242, 1665–1670 (1988).
Sun, J. et al. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80, 1850–1862 (2006).
Blunt, T. et al. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc. Natl Acad. Sci. USA 93, 10285–10290 (1996).
McCune, J. M. et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241, 1632–1639 (1988). This is the first report to describe the engraftment of scid mice with human thymus and liver tissue to establish the scid -hu–Thy/Liv mouse model.
Namikawa, R., Kaneshima, H., Lieberman, M., Weissman, I. L. & McCune, J. M. Infection of the SCID-hu mouse by HIV-1. Science 242, 1684–1686 (1988). This study demonstrates productive HIV-1 infection and CD4+ T cell depletion in human tissues of hu- scid mice.
Aldrovandi, G. M. et al. The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 (1993).
Bonyhadi, M. L. et al. HIV induces thymus depletion in vivo. Nature 363, 728–732 (1993).
Stanley, S. K. et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178, 1151–1163 (1993).
Jenkins, M., Hanley, M. B., Moreno, M. B., Wieder, E. & McCune, J. M. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood 91, 2672–2678 (1998).
McCune, J. M., Namikawa, R., Shih, C. C., Rabin, L. & Kaneshima, H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science 247, 564–566 (1990).
Shih, C. C. et al. Postexposure prophylaxis with zidovudine suppresses human immunodeficiency virus type 1 infection in SCID-hu mice in a time-dependent manner. J. Infect. Dis. 163, 625–627 (1991).
Nonoyama, S., Smith, F. O., Bernstein, I. D. & Ochs, H. D. Strain-dependent leakiness of mice with severe combined immune deficiency. J. Immunol. 150, 3817–3824 (1993).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Mosier, D. E., Gulizia, R. J., Baird, S. M. & Wilson, D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335, 256–259 (1988). This article describes the generation of scid -hu–PBL mice by engraftment of scid mice with human PBLs.
Hoffmann-Fezer, G., Gall, C., Zengerle, U., Kranz, B. & Thierfelder, S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood 81, 3440–3448 (1993).
Mosier, D. E. et al. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251, 791–794 (1991).
Mosier, D. E., Gulizia, R. J., MacIsaac, P. D., Torbett, B. E. & Levy, J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260, 689–692 (1993).
Gauduin, M. C. et al. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nature Med. 3, 1389–1393 (1997).
Parren, P. W. et al. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9, F1–6 (1995).
Safrit, J. T. et al. hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS 7, 15–21 (1993).
Delhem, N. et al. Primary Th1 cell immunization against HIVgp160 in SCID-hu mice coengrafted with peripheral blood lymphocytes and skin. J. Immunol. 161, 2060–2069 (1998).
Mosier, D. E., Gulizia, R. J., MacIsaac, P. D., Corey, L. & Greenberg, P. D. Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc. Natl Acad. Sci. USA 90, 2443–2447 (1993).
Lapenta, C. et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J. Exp. Med. 198, 361–367 (2003).
Yoshida, A. et al. Induction of protective immune responses against R5 human immunodeficiency virus type 1 (HIV-1) infection in hu-PBL-SCID mice by intrasplenic immunization with HIV-1-pulsed dendritic cells: possible involvement of a novel factor of human CD4+ T-cell origin. J. Virol. 77, 8719–8728 (2003).
Shultz, L. D. et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 (1995).
Prochazka, M., Gaskins, H. R., Shultz, L. D. & Leiter, E. H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc. Natl Acad. Sci. USA 89, 3290–3294 (1992).
Ito, M. et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002). This is one of the first reports to describe the development of a human immune system in NOD scid Il2gr−/− mice following engraftment with human CD34+ stem cells derived from cord blood.
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005). This is an early study showing the development of a nearly complete human immune system following the engraftment of NOD scid Il2rg−/− mice with human CD34+ stem cells derived from peripheral blood.
McDermott, S. P., Eppert, K., Lechman, E. R., Doedens, M. & Dick, J. E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116, 193–200 (2010).
Brehm, M. A. et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clin. Immunol. 135, 84–98 (2010).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106, 1565–1573 (2005).
Watanabe, S. et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγnull mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109, 212–218 (2007).
Dash, P. K. et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci. 31, 3148–3157 (2011).
Gorantla, S. et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am. J. Pathol. 177, 2938–2949 (2010).
Holt, N. et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nature Biotech. 28, 839–847 (2010).
Joseph, A. et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J. Virol. 84, 6645–6653 (2010).
Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134, 577–586 (2008).
Balazs, A. B. et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81–84 (2011).
Melkus, M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature Med. 12, 1316–1322 (2006). This paper is the first to describe the generation of BLT mice by the transplantation of CD34+ stem cells into NOD scid mice that had previously been implanted with human thymus and liver tissues.
Wege, A. K., Melkus, M. W., Denton, P. W., Estes, J. D. & Garcia, J. V. Functional and phenotypic characterization of the humanized BLT mouse model. Curr. Top. Microbiol. Immunol. 324, 149–165 (2008).
Brainard, D. M. et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83, 7305–7321 (2009).
Denton, P. W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Denton, P. W. et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE 5, e8829 (2010).
Goldman, J. P. et al. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor γ chain. Br. J. Haematol. 103, 335–342 (1998). This research demonstrates efficient engraftment of human CD34+ stem cells in Rag2−/−Il2rg−/− mice, and shows the advantages of these mice over scid models.
Baenziger, S. et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. Proc. Natl Acad. Sci. USA 103, 15951–15956 (2006).
Berges, B. K., Wheat, W. H., Palmer, B. E., Connick, E. & Akkina, R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−γc−/− (RAG-hu) mouse model. Retrovirology 3, 76 (2006).
Choudhary, S. K. et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−γc−/− mouse. J. Virol. 83, 8254–8258 (2009).
Neff, C. P. et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci. Transl. Med. 3, 66ra6 (2011).
Van Duyne, R. et al. Effect of transcription peptide inhibitors on HIV-1 replication. Virology 376, 308–322 (2008).
Hofer, U. et al. RAG2−/−γc−/− mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J. Virol. 82, 12145–12153 (2008).
Akkina, R. et al. Humanized Rag1−/−γc−/− mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS ONE 6, e20169 (2011).
Berges, B. K., Akkina, S. R., Folkvord, J. M., Connick, E. & Akkina, R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/−γc−/− (RAG-hu) mice. Virology 373, 342–351 (2008).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329, 1168–1174 (2010).
Doulatov, S., Notta, F., Laurenti, E. & Dick, J. E. Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 (2012).
Takizawa, H., Boettcher, S. & Manz, M. G. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119, 2991–3002 (2012).
Garcia, S. & Freitas, A. A. Humanized mice: current states and perspectives. Immunol. Lett. 146, 1–7 (2012).
Rathinam, C. et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood 118, 3119–3128 (2011).
Chahroudi, A., Bosinger, S. E., Vanderford, T. H., Paiardini, M. & Silvestri, G. Natural SIV hosts: showing AIDS the door. Science 335, 1188–1193 (2012).
Hirsch, V. M., Olmsted, R. A., Murphey-Corb, M., Purcell, R. H. & Johnson, P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339, 389–392 (1989).
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998). This work identifies the GALT of rhesus macaques as a major site of SIV replication and CD4+ T cell depletion.
Boyson, J. E. et al. The MHC class I genes of the rhesus monkey: different evolutionary histories of the MHC class I and II genes in primates. J. Immunol. 156, 4656–4665 (1996).
Otting, N. et al. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc. Natl Acad. Sci. USA 102, 1626–1631 (2005).
Loffredo, J. T., Valentine, L. E. & Watkins, D. I. in HIV Molecular Immunology 2006/2007 (eds Korber, B.T. et al.) 29–51 (Los Alamos National Laboratory, 2007).
Mothe, B. R. et al. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77, 2736–2740 (2003).
Yant, L. J. et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80, 5074–5077 (2006).
Loffredo, J. T. et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81, 8827–8832 (2007).
Stremlau, M. et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004). This investigation pinpoints TRIM5α as the cellular protein that accounts for the post-entry block to HIV-1 infection in cells from macaques and other Old World monkeys.
Newman, R. M. et al. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5α. Proc. Natl Acad. Sci. USA 103, 19134–19139 (2006).
Lim, S.-Y. et al. TRIM5α modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6, e1000738 (2010).
Fenizia, C. et al. TRIM5α does not affect simian immunodeficiency virus SIVmac251 replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J. Virol. 85, 12399–12409 (2011).
Kirmaier, A. et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8, e1000462 (2010). This study finds that differences in susceptibility to TRIM5 variants account for the highly variable outcome of SIVsmE543-3 infection in rhesus macaques.
Marthas, M. L., Lu, D., Penedo, M. C. T., Hendrickx, A. G. & Miller, C. J. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retroviruses 17, 1455–1466 (2001).
Ling, B. et al. SIVmac pathogenesis in rhesus macaques of Chinese and Indian orgin compared with primary HIV infections in humans. AIDS 16, 1489–1496 (2002).
Reimann, K. A. et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgous macaques and associated with early T-lymphocyte responses. J. Virol. 79, 8878–8885 (2005).
Yamamoto, H., Kawada, M., Takeda, A., Igarashi, H. & Matano, T. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE 2, e540 (2007).
Karl, J. A. et al. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 60, 37–46 (2008).
Naruse, T. K. et al. Diversity of MHC class I genes in Burmese-origin rhesus macaques. Immunogenetics 62, 601–611 (2010).
Klatt, N. R. et al. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J. Virol. 86, 1203–1213 (2012).
Batten, C. J. et al. Comparative evaluation of simian, simian–human, and human immunodeficiency virus infection in pigtail macaque (Macaca nemestrina) model. AIDS Res. Hum. Retroviruses 22, 580–588 (2006).
Fernandez, C. S. et al. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics 63, 511–521 (2011).
Smith, M. Z. et al. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J. Virol. 79, 684–695 (2005).
Liao, C. H., Kuang, Y. Q., Liu, H. L., Zheng, Y. T. & Su, B. A novel fusion gene, TRIM5-cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS 21 (Suppl. 8), S19–S26 (2007). This investigation is the first to show that pig-tailed macaques express a TRIM5–cyclophilin A fusion protein that does not restrict HIV-1, providing a molecular basis for the susceptibility of pig-tailed macaques to HIV-1 infection.
Wilson, S. J. et al. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl Acad. Sci. USA 105, 3557–3562 (2008).
Virgen, C. A., Kratovac, Z., Bieniasz, P. D. & Hatziioannou, T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl Acad. Sci. USA 105, 3563–3568 (2008).
Newman, R. M. et al. Evolution of a TRIM5-CypA spice isoform in Old World monkeys. PLoS Pathog. 4, e1000003 (2008).
Brennan, G., Kozyrev, Y. & Hu, S.-L. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl Acad. Sci. USA 105, 3569–3574 (2008).
Hatziioannou, T. et al. A macaque model of HIV-1 infection. Proc. Natl Acad. Sci. USA 106, 4425–4429 (2009). This is an important proof-of-concept study demonstrating that a minimally modified strain of HIV-1, differing from HIV-1 only in vif , can replicate in pig-tailed macaques and is sensitive to a combination of antiretroviral drugs used to treat HIV-1 infection in humans.
Igarashi, T. et al. Human immunodeficency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. J. Virol. 81, 11549–11552 (2007).
Pendley, C. J. et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics 60, 339–351 (2008).
Budde, M. L. et al. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics 62, 773–780 (2010).
Dietrich, E. A. et al. Variable prevalence and functional diversity of the antiretroviral restriction factor TRIMCyp in Macaca fascicularis. J. Virol. 85, 9956–9963 (2011).
Krebs, K. C., Jin, Z., Rudersdorf, R., Hughes, A. L. & O'Connor, D. H. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J. Immunol. 175, 5230–5239 (2005).
Wiseman, R. W. et al. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J. Virol. 81, 349–361 (2007).
O'Connor, S. L. et al. MHC heterozygote advantage in simian immunodeficiency virus-infected Mauritian cynomolgus macaques. Sci. Transl. Med. 2, 22ra18 (2010). This research makes elegant use of the limited number of MHC haplotypes in Mauritian cynomolgus macaques to demonstrate the heterozygote advantage in the control of SIV infection.
Daniel, M. D. et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228, 1201–1204 (1985). This is the first report to describe the isolation of SIV from rhesus macaques.
Mansfield, K. G., Lerch, N. W., Gardner, M. B. & Lackner, A. A. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 24, 116–122 (1995).
Apetrei, C. et al. Kuru experiments triggered the emergence of pathogenic SIVmac . AIDS 20, 317–321 (2006).
Keele, B. F. et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206, 1117–1134 (2009).
Kestler, H. et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248, 1109–1112 (1990). This is the first study to show that an infectious molecular clone of SIV can cause AIDS in rhesus macaques.
Hirsch, V. et al. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71, 1608–1620 (1997).
Reynolds, M. R. et al. Macaques vaccinate with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205, 2537–2550 (2008).
Wyand, M. S. et al. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73, 8356–8363 (1999).
Barouch, D. H. et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–94 (2012).
Reynolds, M. R. et al. The TRIM5α genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J. Virol. 85, 9637–9640 (2011).
Riddick, N. E. et al. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 6, e1001064 (2010).
Thakallapally, R., Rose, P., Vasil, S., Pillai, S. & Kuiken, C. L. in Human Retroviruses and AIDS ( eds Kuiken, C. L. et al.) 506–516 (Theoretical Biology and Biophysics Group, 1999).
Reimann, K. A. et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70, 3198–3206 (1996). This report describes the passage and initial isolation of the highly pathogenic chimaera SHIV89.6P.
Joag, S. V. et al. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70, 3189–3197 (1996).
Igarashi, T. et al. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl Acad. Sci. USA 96, 14049–14054 (1999).
Feinberg, M. B. & Moore, J. P. AIDS vaccine models: challenging challenge viruses. Nature Med. 8, 207–210 (2002).
Nishimura, Y. et al. Higly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl Acad. Sci. USA 101, 12324–12329 (2004).
Harouse, J. M., Gettie, A., Tan, R. C., Blanchard, J. & Cheng-Mayer, C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284, 816–819 (1999). This article describes the first pathogenic SHIV found to use CCR5 as a co-receptor.
Veazey, R. S. et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature 483, 99–102 (2005).
Ng, C. T. et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nature Med. 16, 1117–1119 (2010).
Hessel, A. J. et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–105 (2007).
Shakirzyanova, M. et al. Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N . J. Virol. 86, 9432–9442 (2012).
Nishimura, Y. et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J. Virol. 84, 4769–4781 (2010).
Song, R. J. et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication-competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C env. J. Virol. 80, 8729–8738 (2006).
Ambrose, Z. et al. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J. Virol. 78, 13553–13561 (2004).
Uberla, K. et al. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc. Natl Acad. Sci. USA 92, 8210–8214 (1995).
Ambrose, Z. et al. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J. Virol. 81, 12145–12155 (2007).
North, T. W. et al. Suppression of virus load by highly active antiretroviral therapy in rhesus macaques infected with a recombinant simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. J. Virol. 79, 7349–7354 (2005).
Kearney, M. et al. Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy. J. Virol. 85, 1067–1076 (2011).
North, T. W. et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84, 2913–2922 (2010).
Ishimatsu, M. et al. Construction of a novel SHIV having an HIV-1-derived protease gene and its infection to rhesus macaques: a useful tool for in vivo efficacy tests of protease inhibitors. Microbes Infect. 9, 475–482 (2007).
Smith, J. M. et al. Generation of a dual RT Env SHIV that is infectious in rhesus macaques. J. Med. Primatol. 39, 213–223 (2010).
Thippeshappa, R. et al. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1. J. Virol. 85, 3767–3779 (2011).
McNatt, M. W. et al. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5, 1–12 (2009).
Sawyer, S. L., Wu, L. I., Emerman, M. & Malik, H. S. Positive selection of primate TRIM5α identifies a critical species-specific retroviral restriction domain. Proc. Natl Acad. Sci. USA 102, 2832–2837 (2005).
Sawyer, S. L., Emerman, M. & Malik, H. S. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2, 1278–1285 (2004).
Sayah, D. M., Sokolskaja, E., Berthoux, L. & Luban, J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430, 569–573 (2004).
Stremlau, M. et al. Species recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl Acad. Sci. USA 103, 5514–5519 (2006).
Sebastian, S. & Luban, J. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2, 40 (2005).
Chiu, Y. L. & Greene, W. C. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 26, 317–353 (2008).
Schafer, A., Bogerd, H. P. & Cullen, B. R. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virology 328, 163–168 (2004).
Svarovskaia, E. S. et al. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J. Biol. Chem. 279, 35822–35828 (2004).
Zennou, V., Perez-Caballero, D., Gottlinger, H. & Bieniasz, P. D. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 78, 12058–12061 (2004).
Sheehy, A. M., Gaddis, N. C., Choi, J. D. & Malim, M. H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002). This work identifes APOBEC3G (also known as CEM15) as the first known restriction factor for HIV-1; this protein family is now recognized as an important block to HIV-1 replication in macaques.
Harris, R. S. et al. DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809 (2003).
Mangeat, B. et al. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424, 99–103 (2003).
Zhang, H. et al. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424, 94–98 (2003).
Marin, M., Rose, K. M., Kozak, S. L. & Kabat, D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nature Med. 9, 1398–1403 (2003).
Sheehy, A. M., Gaddis, N. C. & Malim, M. H. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nature Med. 9, 1404–1407 (2003).
Yu, X. et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 vif-cul5-SCF complex. Science 302, 1056–1060 (2003).
Mariani, R. et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114, 21–31 (2003).
Virgen, C. A. & Hatziioannou, T. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. J. Virol. 81, 13932–13937 (2007).
Neil, S. J. D., Zang, T. & Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 1–8 (2008). References 161 and 162 identify tetherin as a restriction factor for HIV-1.
Perez-Caballero, D. et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139, 499–511 (2009).
Fitzpatrick, K. et al. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6, e1000701 (2010).
Hammonds, J., Wang, J.-J., Yi, H. & Spearman, P. Immunoelectron microscopic evidence for tetherin/BST2 as a the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6, e1000749 (2010).
Jia, B. et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5, e1000429 (2009).
Zhang, F., Lanford, W., Ng, M., Bieniasz, P. & Hatziioannou, T. SIV Nef proteins antagonize tetherin by AP-2-dependent removal from sites of virion budding. 18th Conference on Retroviruses and Opportunistic Infections Abstract 85 (2011).
Sauter, D. et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6, 409–421 (2009).
Hauser, H. et al. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7, 51 (2010).
LeTortorec, A. & Neil, S. J. D. Antagonism and intracellular sequestration of human tetherin by the HIV-2 envelope glycoprotein. J. Virol. 83, 11966–11978 (2009).
Douglas, J. L. et al. Vpu directs the degradation of the human immunodeficiency virus retriction factor BST-2/tetherin via a βTrCP-dependent mechanism. J. Virol. 83, 7931–7947 (2009).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 (2011).
Powell, R. D., Holland, P. J., Hollis, T. & Perrino, F. W. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J. Biol. Chem. 286, 43596–43600 (2011).
Hrecka, K. et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011).
Laguette, N. et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011).
Martin-Serrano, J. & Neil, S. J. D. Host factors involved in retroviral budding and release. Nature Rev. Microbiol. 9, 519–531 (2011).
Pedersen, N. C., Ho, E. W., Brown, M. L. & Yamamoto, J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235, 790–793 (1987).
Torten, M. et al. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 65, 2225–2230 (1991).
Dow, S. W., Poss, M. L. & Hoover, E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 3, 658–668 (1990).
Podell, M., March, P. A., Buck, W. R. & Mathes, L. E. The feline model of neuroAIDS: understanding the progression towards AIDS dementia. J. Psychopharmacol. 14, 205–213 (2000).
Wongsrikeao, P., Saenz, D., Rinkoski, T., Otoi, T. & Poeschla, E. Antiviral restriction factor transgenesis in the domestic cat. Nature Methods 8, 853–859 (2011).
Arai, M., Earl, D. D. & Yamamoto, J. K. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet. Immunol. Immunopathol. 85, 189–204 (2002).
Hayes, K. A., Lafrado, L. J., Erickson, J. G., Marr, J. M. & Mathes, L. E. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J. Acquir. Immune Def. Syndr. 6, 127–134 (1993).
Hayes, K. A., Wilkinson, J. G., Frick, R., Francke, S. & Mathes, L. E. Early suppression of viremia by ZDV does not alter the spread of feline immunodeficiency virus infection in cats. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9, 114–122 (1995).
Smyth, N. R. et al. Effect of 3'azido-2',3'-deoxythymidine (AZT) on experimental feline immunodeficiency virus infection in domestic cats. Res. Vet. Sci. 57, 220–224 (1994).
Egberink, H. et al. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc. Natl Acad. Sci. USA 87, 3087–3091 (1990).
Hartmann, K. et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet. Immunol. Immunopathol. 35, 167–175 (1992).
Puras Lutzke, R. A., Eppens, N. A., Weber, P. A., Houghten, R. A. & Plasterk, R. H. Identification of a hexapeptide inhibitor of the human immunodeficiency virus integrase protein by using a combinatorial chemical library. Proc. Natl Acad. Sci. USA 92, 11456–11460 (1995).
Mazumder, A. et al. Inhibition of the human immunodeficiency virus type 1 integrase by guanosine quartet structures. Biochemistry 35, 13762–13771 (1996).
Savarino, A. et al. Human immunodeficiency virus integrase inhibitors efficiently suppress feline immunodeficiency virus replication in vitro and provide a rationale to redesign antiretroviral treatment for feline AIDS. Retrovirology 4, 79 (2007).
Auwerx, J., Esnouf, R., De Clercq, E. & Balzarini, J. Susceptibility of feline immunodeficiency virus/human immunodeficiency virus type 1 reverse transcriptase chimeras to non-nucleoside RT inhibitors. Mol. Pharmacol. 65, 244–251 (2004).
Slee, D. H. et al. Selectivity in the inhibition of HIV and FIV protease: inhibitory and mechanistic studies of pyrrolidine-containing α-keto amide and hydroxyethylamine core structures. J. Am. Chem. Soc. 117, 11867–11878 (1995).
Sparger, E. E. in In Vivo Models of HIV Disease and Control (eds Friedman, H., Specter, S. & Bendinelli, M.) 149–237 (Springer, 2006).
Shimojima, M. et al. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 303, 1192–1195 (2004).
Dean, G. A., Reubel, G. H., Moore, P. F. & Pedersen, N. C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 70, 5165–5169 (1996).
Rud, E. W. et al. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J. Gen. Virol. 75, 529–543 (1994).
Benveniste, R. E. et al. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV/LAV and other lentiviruses. J. Virol. 60, 483–490 (1986).
Morton, W. R. et al. Transmission of the simian immunodeficiency virus SIVmne in macaques and baboons. J. Med. Primatol. 18, 237–245 (1989).
Rudensey, L. M., Kimata, J. T., Long, E. M., Chackerian, B. & Overbaugh, J. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVmne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72, 209–217 (1998).
Chackerian, B., Rudensey, L. M. & Overbaugh, J. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71, 7719–7727 (1997).
Kimata, J. T., Kuller, L., Anderson, D. B., Dailey, P. & Overbaugh, J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nature Med. 5, 535–541 (1999).
Fultz, P. N., McClure, H. M., Anderson, D. C. & Switzer, W. M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res. Hum. Retroviruses 5, 397–409 (1989).
Smith, D. G. & McDonough, J. Mitochondrial DNA variation in Chinese and Indian rhesus macaques (Macaca mulatta). Am. J. Pirmatol. 65, 1–25 (2005).
Hernandez, R. D. et al. Demographic histories and patterns of linkage disequilibrium in Chinese and Indian rhesus macaques. Science 316, 240–243 (2007).
Morales, J. C. & Melnick, D. J. Phylogenetic relationships of the macaques (Ceropithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal gens. J. Hum. Evol. 34, 1–23 (1998).
Wolfheim, J. H. Primates of the World: Distribution, Abundance, and Conservation (Univ. of Washington Press, 1983).
Acknowledgements
The authors are grateful to P. Bieniasz and R. Desrosiers for critically reviewing this manuscript. The authors also thank P. Marx for sharing the data included in table 1, and A. Halper-Stromberg and R. Liberatore for help with figure 1 and lessons in mouse anatomy. The authors acknowledge and regret that a number of important studies are not cited owing to space constraints. T.H. is supported by US Department of Health and Human Services Public Health Service (PHS) grants AI078788 and AI093255. D.T.E. is supported by PHS grants AI087498, AI095098, AI098485 and RR000168/OD011103, and is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- ESCRT
-
(Endosomal sorting complex required for transport). A conserved cellular machinery for the sorting of ubiquitylated cargo proteins into vesicles and the subsequent scission of the membrane neck. The recruitment of ESCRT proteins through retroviral late domains is essential for viral budding.
- Proviruses
-
The DNA forms of retroviral genomes that are integrated into host chromosomal DNA.
- Haematopoietic (CD34+) progenitor stem cells
-
A population of multipotent cells that can differentiate into any type of blood cell, including the major cell lineages of the immune system. These cells are typically defined by the cell surface marker CD34.
- Natural killer cells
-
Cytotoxic lymphocytes that do not express the B cell or T cell receptor and are capable of responding to virus-infected cells or tumour cells without prior antigenic stimulation.
- Passive immunization
-
The transfer of immunoglobulins.
- Complement
-
A system of >25 serum proteins that is activated by a number of triggers responding to foreign antigen on the surface of a cell; complement activation results in a proteolytic cascade leading to cell lysis or enhanced phagocytosis.
- Gut-associated lymphoid tissue
-
(GALT). Secondary lymphoid tissue associated with the digestive tract, including the Peyer's patches, mesenteric lymph nodes and the appendix. For the purpose of this Review, the GALT also refers to effector sites of the lamina propria and intestinal epithelium that are rich in memory lymphocytes which support HIV and simian immunodeficiency virus (SIV) replication.
- Central memory CD4+ T cells
-
Antigen-experienced resting T cells that express cell surface receptors which are required for homing to secondary lymphoid organs. These cells are generally long-lived and can serve as the precursors for effector T cells during recall responses.
- Effector memory CD4+ T cells
-
Terminally differentiated T cells that lack lymph node-homing receptors but express receptors that enable the cells to home to inflamed tissues. Effector memory T cells can exert immediate effector functions without the need for further differentiation.
Rights and permissions
About this article
Cite this article
Hatziioannou, T., Evans, D. Animal models for HIV/AIDS research. Nat Rev Microbiol 10, 852–867 (2012). https://doi.org/10.1038/nrmicro2911
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro2911
This article is cited by
-
Microphysiological model reveals the promise of memory-like natural killer cell immunotherapy for HIV± cancer
Nature Communications (2023)
-
Subsequent malaria enhances virus-specific T cell immunity in SIV-infected Chinese rhesus macaques
Cell Communication and Signaling (2022)
-
Neuroinflammation in HIV-associated depression: evidence and future perspectives
Molecular Psychiatry (2022)
-
Brain tissue transcriptomic analysis of SIV-infected macaques identifies several altered metabolic pathways linked to neuropathogenesis and poly (ADP-ribose) polymerases (PARPs) as potential therapeutic targets
Journal of NeuroVirology (2021)
-
Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders
Fluids and Barriers of the CNS (2020)