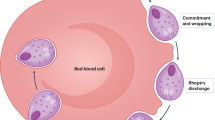
Key Points
-
Malaria is the most deadly parasitic infection of humans, killing up to 1 million people per year. No vaccine is currently available, and the development of drug-resistant Plasmodium spp. is of increasing concern.
-
The first phase of infection, the pre-erythrocytic (PE) phase, is clinically asymptomatic. Only after parasite replication in the liver and infection of large numbers of erythrocytes do symptoms arise.
-
The PE phase comprises sporozoites (the infectious stage) and the liver stages. Once injected by a mosquito, the sporozoites can remain in the skin, be transported in lymph vessels to draining lymph nodes or travel through the bloodstream to the liver. In the liver, sporozoites undergo an elaborate replication and developmental programme and transform into the merozoites that are released from the liver to infect erythrocytes.
-
The PE phase of infection is a formidable window of opportunity for therapeutic interventions owing to the small number of parasites present. Thus, targeting this 'bottleneck' of Plasmodium spp. infection with vaccines is an attractive strategy.
-
Live attenuated parasites mimicking the PE phase of infection can be used as vaccines. Attenuation is achieved by radiation, genetic alterations or drug-mediated developmental arrest.
Abstract
Malaria, which is caused by Plasmodium spp., starts with an asymptomatic phase, during which sporozoites, the parasite form that is injected into the skin by a mosquito, develop into merozoites, the form that infects erythrocytes. This pre-erythrocytic phase is still the most enigmatic in the parasite life cycle, but has long been recognized as an attractive vaccination target. In this Review, we present what has been learned in recent years about the natural history of the pre-erythrocytic stages, mainly using intravital imaging in rodents. We also consider how this new knowledge is in turn changing our understanding of the immune response mounted by the host against the pre-erythrocytic forms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
08 October 2013
In Box 3 of the above article (page 709) the sentence “Recently, it was found that five intravenous injections of >105 cryopreserved RAS protected all six tested patients against a challenge by bites of five mosquitoes 3 weeks after the last immunization dose, whereas four intravenous immunizations protected only three of nine patients139” should have read “Recently, it was found that five intravenous injections of >105 cryopreserved RAS protected all six tested patients against a challenge by bites of five mosquitoes 3 weeks after the last immunization dose, whereas four intravenous immunizations protected only six of nine patients139.” The authors apologize to readers for any misunderstanding caused. This has now been corrected online.
References
Carter, R. & Mendis, K. N. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 15, 564–594 (2002).
WHO. Malaria fact sheet. WHO Media Center [online], (2013).
Mbogo, C. M. et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am. J. Trop. Med. Hyg. 68, 734–742 (2003).
Mulligan, H. W., Russell, P. F. & Mohan, B. N. Active immunization of fowls against Plasmodium gallinaceum by injections of killed homologous sporozoites. J. Malaria Inst. India 4, 25–34 (1941).
Nussenzweig, R. S., Vanderberg, J., Most, H. & Orton, C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216, 160–162 (1967). The first vaccination of mice against sporozoite challenge.
Clyde, D. F., Most, H., McCarthy, V. C. & Vanderberg, J. P. Immunization of man against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266, 169–177 (1973). The first vaccination of humans against malaria.
Laveran, A. Un nouveau parasite trouvé dans le sang des malades atteints de fièvre palustre: origine parasitaire des accidents de l'impaludisme. Bull. Mem. Soc. Med. Hop. Paris 17, 158–164 (in French) (1881).
Ross, R. On some peculiar pigmented cells found in two mosquitos fed on malaria blood. Br. Med. J. 2, 1786–1788 (1897).
Grassi, B., Bignami, A. & Bastianelli, G. Ulteriori ricerche sul ciclo dei parassiti malarici umani nel corpo del zanzarone. Atti R. Accad. Lincei 8, 21–28 (1899) (in Italian).
Huff, C. G. Life cycle of malarial parasites. Annu. Rev. Microbiol. 1, 43–60 (1947).
Shortt, H. E., Garnham, P. C. C. & Malamos, B. The pre-erythrocytic stage of mammalian malaria. Br. Med. J. 1, 192–194 (1948). The first demonstration that sporozoites of Plasmodium spp. which infect mammals transform in the liver.
Krotoski, W. A. et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am. J. Trop. Med. Hyg. 31, 1291–1293 (1982).
Markus, M. B. The hypnozoite concept, with particular reference to malaria. Parasitol. Res. 108, 247–252 (2011).
Markus, M. B. Dormancy in mammalian malaria. Trends Parasitol. 28, 39–45 (2012).
Vincke, I. H. & Lips, M. Un nouveau Plasmodium d'un rongeur sauvage du Congo: Plasmodium berghei n. sp. Ann. Soc. Belge Med. Trop. 28, 97–104 (1948) (in French).
Yoeli, M., Most, H. & Boné, G. Plasmodium berghei: cyclical transmissions by experimentally infected Anopheles quadrimaculatus. Science 144, 1580–1581 (1964).
Yoeli, M., Vanderberg, J., Upmanis, R. S. & Most, H. Primary tissue phase of Plasmodium berghei in different experimental hosts. Nature 208, 903 (1965). The first observation of P. berghei schizonts in the liver of rodents.
van Dijk, M. R., Janse, C. J. & Waters, A. P. Expression of a Plasmodium gene introduced into subtelomeric regions of Plasmodium berghei chromosomes. Science 271, 662–665 (1996). The first genetic transformation by homologous recombination in P. berghei erythrocytic stages.
Vanderberg, J. P. Studies on the motility of Plasmodium sporozoites. J. Protozool. 21, 527–537 (1974).
Vanderberg, J. P., Chew, S. & Stewart, M. J. Plasmodium sporozoite interactions with macrophages in vitro: a videomicroscopic analysis. J. Protozool. 37, 528–536 (1990). The first observation of Plasmodium sp.sporozoites traversing host cells in vitro.
Mota, M. M. et al. Migration of Plasmodium sporozoites through cells before infection. Science 291, 141–144 (2001). A study that shows the membrane-wounding activity of Plasmodium spp. sporozoites.
Hollingdale, M. R., Leef, J. L., McCullough, M. & Beaudoin, R. L. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei from sporozoites. Science 213, 1021–1022 (1981).
Mazier, D. et al. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 (1985).
Ponnudurai, T., Lensen, A. H., van Gemert, G. J., Bolmer, M. G. & Meuwissen, J. H. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans. R. Soc. Trop. Med. Hyg. 85, 175–180 (1991).
Medica, D. L. & Sinnis, P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 73, 4363–4369 (2005).
Beier, J. C., Davis, J. R., Vaughan, J. A., Noden, B. H. & Beier, M. S. Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi. Am. J. Trop. Med. Hyg. 44, 564–570 (1991).
Frischknecht, F. et al. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell. Microbiol. 6, 687–694 (2004).
Jin, Y., Kebaier, C. & Vanderberg, J. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 75, 5532–5539 (2007).
Sidjanski, S. & Vanderberg, J. P. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am. J. Trop. Med. Hyg. 57, 426–429 (1997). Work showing that most sporozoites are deposited into the skin when a mosquito bites.
Matsuoka, H., Yoshida, S., Hirai, M. & Ishii, A. A rodent malaria, Plasmodium berghei, is experimentally transmitted to mice by merely probing of infective mosquito, Anopheles stephensi. Parasitol. Int. 51, 17–23 (2002).
Vanderberg, J. P. & Frevert, U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34, 991–996 (2004). The first imaging of sporozoite gliding motility in the skin of mice.
Amino, R. et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nature Med. 12, 220–224 (2006). An article describing the tripartite fate of sporozoites inoculated into the skin.
Amino, R. et al. Imaging malaria sporozoites in the dermis of the mammalian host. Nature Protoc. 2, 1705–1712 (2007).
Yamauchi, L. M., Coppi, A., Snounou, G. & Sinnis, P. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9, 1215–1222 (2007).
Xu, W.-Y., Wang, X.-X., Qi, J., Duan, J.-H. & Huang, F.-S. Plasmodium yoelii: influence of immune modulators on the development of the liver stage. Exp. Parasitol. 126, 254–258 (2010).
Ellis, J. et al. Cloning and expression in E. coli of the malarial sporozoite surface antigen gene from Plasmodium knowlesi. Nature 302, 536–538 (1983). The first cloning of a Plasmodium spp. gene.
Pradel, G., Garapaty, S. & Frevert, U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol. Microbiol. 45, 637–651 (2002).
Suarez, J. E. et al. Plasmodium falciparum circumsporozoite (CS) protein peptides specifically bind to HepG2 cells. Vaccine 19, 4487–4495 (2001).
Rathore, D., Sacci, J. B., de la Vega, P. & McCutchan, T. F. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 277, 7092–7098 (2002).
Cerami, C. et al. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70, 1021–1033 (1992).
Frevert, U. et al. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177, 1287–1298 (1993).
Sinnis, P. et al. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med. 180, 297–306 (1994).
Coppi, A. et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 208, 341–356 (2011).
Frevert, U. et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3, e192 (2005).
Mota, M. M., Hafalla, J. C. R. & Rodriguez, A. Migration through host cells activates Plasmodium sporozoites for infection. Nature Med. 8, 1318–1322 (2002).
Carrolo, M. et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nature Med. 9, 1363–1369 (2003).
Kaushansky, A. & Kappe, S. H. I. The crucial role of hepatocyte growth factor receptor during liver-stage infection is not conserved among Plasmodium species. Nature Med. 17, 1180–1181 (2011).
Ishino, T., Yano, K., Chinzei, Y. & Yuda, M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2, e4 (2004). The generation of the first Plasmodium spp. sporozoites deficient in CT.
Ishino, T., Chinzei, Y. & Yuda, M. A. Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell. Microbiol. 7, 199–208 (2005).
Yuda, M. & Ishino, T. Liver invasion by malarial parasites — how do malarial parasites break through the host barrier? Cell. Microbiol. 6, 1119–1125 (2004).
Amino, R. et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3, 88–96 (2008).
Tavares, J. et al. Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 210, 905–915 (2013).
Pradel, G. & Frevert, U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 33, 1154–1165 (2001).
Baer, K. et al. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9, 397–412 (2007).
Coppi, A. et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2, 316–327 (2007).
Frevert, U., Sinnis, P., Esko, J. D. & Nussenzweig, V. Cell surface glycosaminoglycans are not obligatory for Plasmodium berghei sporozoite invasion in vitro. Mol. Biochem. Parasitol. 76, 257–266 (1996).
Coppens, I., Sullivan, D. J. & Prigge, S. T. An update on the rapid advances in malaria parasite cell biology. Trends Parasitol. 26, 305–310 (2010).
Spielmann, T., Montagna, G. N., Hecht, L. & Matuschewski, K. Molecular make-up of the Plasmodium parasitophorous vacuolar membrane. Int. J. Med. Microbiol. 302, 179–186 (2012).
Graewe, S., Stanway, R. R., Rennenberg, A. & Heussler, V. T. Chronicle of a death foretold: Plasmodium liver stage parasites decide on the fate of the host cell. FEMS Microbiol. Rev. 36, 111–130 (2012).
Lim, L. & McFadden, G. I. The evolution, metabolism and functions of the apicoplast. Phil. Trans. R. Soc. 365, 749–763 (2010).
Van de Sand, C. et al. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol. Microbiol. 58, 731–742 (2005).
Rennenberg, A. et al. Exoerythrocytic Plasmodium parasites secrete a cysteine protease inhibitor involved in sporozoite invasion and capable of blocking cell death of host hepatocytes. PLoS Pathog. 6, e1000825 (2010).
Kaushansky, A. et al. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 3, 630–637 (2013).
Sturm, A. et al. Alteration of the parasite plasma membrane and the parasitophorous vacuole membrane during exo-erythrocytic development of malaria parasites. Protist 160, 51–63 (2009).
Schmidt-Christensen, A., Sturm, A., Horstmann, S. & Heussler, V. T. Expression and processing of Plasmodium berghei SERA3 during liver stages. Cell. Microbiol. 10, 1723–1734 (2008).
Graewe, S. et al. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 7, e1002224 (2011).
Frevert, U. Sneaking in through the back entrance: the biology of malaria liver stages. Trends Parasitol. 20, 417–424 (2004).
Sturm, A. et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313, 1287–1290 (2006). The first observation of hepatic merozoite release via merosomes.
Thiberge, S. et al. In vivo imaging of malaria parasites in the murine liver. Nature Protoc. 2, 1811–1818 (2007).
Vaughan, A. M. et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 122, 3618–3628 (2012).
Baer, K., Klotz, C., Kappe, S. H. I., Schnieder, T. & Frevert, U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 3, e171 (2007).
Gueirard, P. et al. Development of the malaria parasite in the skin of the mammalian host. Proc. Natl Acad. Sci. USA 107, 18640–18645 (2010). The first observation of merozoite formation in the skin of mice.
Voza, T., Miller, J. L., Kappe, S. H. I. & Sinnis, P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infect. Immun. 80, 2158–2164 (2012).
Mellor, A. L. & Munn, D. H. Immune privilege: a recurrent theme in immunoregulation? Immunol. Rev. 213, 5–11 (2006).
Frevert, U., Späth, G. F. & Yee, H. Exoerythrocytic development of Plasmodium gallinaceum in the White Leghorn chicken. Int. J. Parasitol. 38, 655–672 (2008).
Silvie, O. et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nature Med. 9, 93–96 (2003).
Boyd, M. F. & Kitchen, S. F. The demonstration of sporozoites in human tissues. Am. J. Trop. Med. Hyg. 19, 27–31 (1939).
Offeddu, V., Thathy, V., Marsh, K. & Matuschewski, K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int. J. Parasitol. 42, 535–548 (2012).
Hoffman, S. L. et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185, 1155–1164 (2002).
Mueller, A.-K., Labaied, M., Kappe, S. H. I. & Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433, 164–167 (2005). The first GAP-induced protection against sporozoite challenge in mice.
Mueller, A.-K. et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl Acad. Sci. USA 102, 3022–3027 (2005).
van Dijk, M. R. et al. Genetically attenuated, 36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl Acad. Sci. USA 102, 12194–12199 (2005).
Labaied, M. et al. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 75, 3758–3768 (2007).
Jobe, O. et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I-dependent interferon-γ-producing CD8+ T cells. J. Infect. Dis. 196, 599–607 (2007).
Mueller, A.-K. et al. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am. J. Pathol. 171, 107–115 (2007).
Tarun, A. S. et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 196, 608–616 (2007).
Vaughan, A. M. et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11, 506–520 (2009).
Pei, Y. et al. Plasmodium pyruvate dehydrogenase activity is only essential for the parasite's progression from liver infection to blood infection. Mol. Microbiol. 75, 957–971 (2010).
Haussig, J. M., Matuschewski, K. & Kooij, T. W. A. Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol. Microbiol. 81, 1511–1525 (2011).
Butler, N. S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011). Work showing that late-arrested liver-stage GAP protect better than RAS.
Falae, A. et al. Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development. J. Biol. Chem. 285, 3282–3288 (2010).
Beaudoin, R. L., Strome, C. P. A., Mitchell, F. & Tubergen, T. A. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp. Parasitol. 42, 1–5 (1977). The first use of DAP to protect against malaria in rodents.
Orjih, A. U., Cochrane, A. H. & Nussenzweig, R. S. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans. R. Soc. Trop. Med. Hyg. 76, 57–61 (1982).
Belnoue, E. et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172, 2487–2495 (2004).
Inoue, M. & Culleton, R. L. The intradermal route for inoculation of sporozoites of rodent malaria parasites for immunological studies. Parasite Immunol. 33, 137–142 (2011).
Friesen, F. et al. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 14, 40ra49 (2010).
Friesen, J. & Matuschewski, K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine 29, 7002–7008 (2011).
Roestenberg, M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361, 468–477 (2009). Validation of the DAP concept in humans.
Roestenberg, M. et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377, 1770–1776 (2011).
Purcell, L. A. et al. Chemically attenuated Plasmodium sporozoites induce specific immune responses, sterile immunity and cross-protection against heterologous challenge. Vaccine 26, 4880–4884 (2008).
Ngonseu, E., Chatterjee, S. & Wery, M. Blocked hepatic-stage parasites and decreased susceptibility to Plasmodium berghei infections in BALB/c mice. Parasitology 117, 419–423 (1998).
Schmidt, N. W., Butler, N. S. & Harty, J. T. Plasmodium–host interactions directly influence the threshold of memory CD8 T cells required for protective immunity. J. Immunol. 186, 5873–5884 (2011).
Yoshida, N., Nussenzweig, R. S., Potocnjak, P., Nussenzweig, V. & Aikawa, M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207, 71–73 (1980). The first demonstration that antibodies to CSP neutralize infectivity.
Stewart, M. J., Nawrot, R. J., Schulman, S. & Vanderberg, J. P. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 51, 859–864 (1986).
Schofield, L. et al. γ-interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330, 664–666 (1987). The first evidence that CD8+ T cells are important for RAS-induced protection in mice.
Weiss, W. R., Sedegah, M., Beaudoin, R. L., Miller, L. H. & Good, M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl Acad. Sci. USA 85, 573–576 (1988).
Romero, P. et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341, 323–326 (1989).
Rodrigues, M. M. et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3, 579–585 (1991).
Tsuji, M., Romero, P., Nussenzweig, R. S. & Zavala, F. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 172, 1353–1357 (1990).
Rénia, L. et al. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J. Immunol. 150, 1471–1478 (1993).
Chakravarty, S., Baldeviano, G. C., Overstreet, M. G. & Zavala, F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require γ interferon for antiparasite activity. Infect. Immun. 76, 3628–3631 (2008).
Butler, N. S., Schmidt, N. W. & Harty, J. T. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J. Immunol. 184, 2528–2538 (2010).
Cockburn, I. A. et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc. Natl Acad. Sci. USA 110, 9090–9095 (2013). The first In vivo imaging of pathogen killing by CD8+ T cells.
Cohen, J., Nussenzweig, V., Nussenzweig, R., Vekemans, J. & Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccines 6, 90–96 (2010).
Luke, T. C. & Hoffman, S. L. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 206, 3803–3808 (2003).
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011).
Nganou-Makamdop, K. & Sauerwein, R. W. Liver or blood-stage arrest during malaria sporozoite immunization: the later the better? Trends Parasitol. 29, 304–310 (2013).
Stoute, J. A. et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336, 86–91 (1997).
Kester, K. E. et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naïve adults at the Walter Reed Army Institute of Research. Vaccine 26, 2191–2202 (2008).
Kester, K. E. et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS, S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200, 337–346 (2009).
Agnandji, S. T. et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 (2011).
Nussenzweig, V., Good, M. F. & Hill, A. V. S. Mixed results for a malaria vaccine. Nature Med. 17, 1560–1561 (2011).
Olotu, A. et al. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 368, 1111–1120 (2013).
Mellouk, S., Lunel, F., Sedegah, M., Beaudoin, R. & Druilhe, P. Protection against malaria induced by irradiated sporozoites. Lancet 335, 721 (1990).
Chatterjee, S., François, G., Druilhe, P., Timperman, G. & Wéry, M. Immunity to Plasmodium berghei exoerythrocytic forms derived from irradiated sporozoites. Parasitol. Res. 82, 297–303 (1996).
Silvie, O. et al. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 24, 221–223 (2002).
Pombo, D. J. et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360, 610–617 (2002).
Bijker, E. M. et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl Acad. Sci. USA 110, 7862–7867 (2013).
Yu, M. et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4, 567–578 (2008). Work showing an essential role for the parasite apicoplast in the late phase of liver-stage development.
Lasonder, E. et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 4, e1000195 (2008).
Tarun, A. S. et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl Acad. Sci. USA 105, 305–310 (2008).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nature Med. 13, 1035–1041 (2007). A study which finds that protective immune responses to sporozoites are generated in skin-draining lymph nodes.
Obeid, M. et al. Skin-draining lymph node priming is sufficient to induce sterile immunity against pre-erythrocytic malaria. EMBO Mol. Med. 5, 250–263 (2013).
Sano, G. et al. Swift development of protective effector functions in naive CD8+ T cells against malaria liver stages. J. Exp. Med. 194, 173–179 (2001).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Cockburn, I. A. et al. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from Plasmodium in vivo. PLoS Pathog. 7, e1001318 (2011). The PEXEL motif is shown to be dispensable for presentation of the CSP antigen.
Cockburn, I. A. et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 6, e1000877 (2010).
Hafalla, J. C. R. et al. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur. J. Immunol. 36, 1179–1186 (2006).
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science http://dx.doi.org/10.1126/science.1241800 (2013). The first demonstrated protection of humans by parenteral injection of RAS.
Nganou-Makamdop, K. et al. Reduced Plasmodium berghei sporozoite liver load associates with low protective efficacy after intradermal immunization. Parasite Immunol. 34, 562–569 (2012).
Voza, T., Kebaier, C. & Vanderberg, J. P. Intradermal immunization of mice with radiation-attenuated sporozoites of Plasmodium yoelii induces effective protective immunity. Malaria J. 9, 362 (2010).
Ploemen, I. H. et al. Plasmodium liver load following parenteral sporozoite administration in rodents. Vaccine 31, 3410–3416 (2013).
Bongfen, S. E., Torgler, R., Romero, J. F., Rénia, L. & Corradin, G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J. Immunol. 178, 7054–7063 (2007).
Spielmann, T. & Gilberger, T.-W. Protein export in malaria parasites: do multiple export motifs add up to multiple export pathways? Trends Parasitol. 26, 6–10 (2010).
Grüring, C. et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe 12, 717–729 (2012).
Singh, A. P. et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell 131, 492–504 (2007).
Ginhoux, F., Ng, L. G. & Merad, M. Understanding the murine cutaneous dendritic cell network to improve intradermal vaccination strategies. Curr. Top. Microbiol. Immunol. 351, 1–24 (2012).
Guilbride, D. L., Gawlinski, P. & Guilbride, P. D. L. Why functional pre-erythrocytic and bloodstage malaria vaccines fail: a meta-analysis of fully protective immunizations and novel immunological model. PLoS ONE 5, e10685 (2010).
Druilhe, P. & Barnwell, J. W. Pre-erythrocytic stage malaria vaccines: time for a change in path. Curr. Opin. Microbiol. 10, 371–378 (2007).
Hill, A. V. S. et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum. Vaccines 6, 78–83 (2010).
Reyes-Sandoval, A. et al. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 187, 1347–1357 (2011).
Kumar, K. A. et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444, 937–940 (2006).
Mauduit, M. et al. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect. Immun. 78, 2182–2188 (2010).
Murphy, S. C., Kas, A., Stone, B. C. & Bevan, M. J. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. Proc. Natl Acad. Sci. USA 110, 6055–6060 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Sterilizing immunity
-
Immunity resulting in parasite clearance from the host.
- Schizonts
-
Multinucleate, intracellular parasite stages originating from a single organism that reproduces by schizogony. Schizonts contain many individual merozoites when mature.
- Hypnozoites
-
Dormant, non-dividing, intrahepatocytic forms of certain Plasmodium species, including Plasmodium vivax and Plasmodium ovale, which infect humans.
- Recurrences
-
New phases of parasite multiplication inside erythrocytes. These recurrences originate from intra-erythrocytic parasite forms (recrudescence) or hypnozoites (relapse).
- Gliding
-
A substrate-dependent type of unicellular motility defined by the lack of cell deformation during movement.
- Parasitophorous vacuole
-
The vacuole inside which the parasite resides on host cell entry and throughout intracellular development.
- Stellate cells
-
Pericytes that are located between a hepatocyte and a sinusoidal endothelial cell; also called Ito cells.
- Kupffer cell
-
A resident macrophage in the liver; these cells mostly double-line the endothelial cell wall inside the sinusoid lumen.
- Apicoplast
-
A relict, non-photosynthetic plastid-like organelle of red-algal origin, inherited from secondary endosymbiosis.
- Immunoprivileged sites
-
Sites in the mammalian body that are characterized by the lack of major histocompatibility complex expression, the absence of phagocytic and antigen-presenting cells, and a potent immunosuppressant microenvironment.
- Subunit vaccination
-
Vaccination using material that is distinct from live parasites (typically, recombinant proteins or virus-vectored antigens).
Rights and permissions
About this article
Cite this article
Ménard, R., Tavares, J., Cockburn, I. et al. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol 11, 701–712 (2013). https://doi.org/10.1038/nrmicro3111
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro3111
This article is cited by
-
Escaping the enemy’s bullets: an update on how malaria parasites evade host immune response
Parasitology Research (2023)
-
Collective migration reveals mechanical flexibility of malaria parasites
Nature Physics (2022)
-
Limited Plasmodium sporozoite gliding motility in the absence of TRAP family adhesins
Malaria Journal (2021)
-
Liposomes for malaria management: the evolution from 1980 to 2020
Malaria Journal (2021)
-
Cryptic Plasmodium chronic infections: was Maurizio Ascoli right?
Malaria Journal (2020)