Key Points
-
Orthobunyaviruses are arthropod-transmitted viruses that are characterized by a tripartite, negative-sense RNA genome. Some viruses in this family are associated with diseases in humans (such as fever and encephalitis) and domesticated animals (including abortion and teratogenic effects in offspring). Schmallenberg virus, which is a recently emerged member of the family, caused a disease outbreak in domesticated animals in Europe in 2012–2013.
-
Viral replication occurs in the cytoplasm of infected cells and viruses mature by budding in the Golgi complex. Although infection of mammalian cells usually results in cell death, replication in arthropod vector cells is not cytopathic and these cells become persistently infected.
-
Viral mRNA synthesis is primed by capped oligonucleotides that are derived from host cell mRNAs in a process that is known as cap snatching. The endonuclease activity that is responsible for generating the primers is contained in the amino-terminal domain of the viral RNA-dependent RNA polymerase protein.
-
The three-dimensional structure of the viral N (nucleocapsid) protein shows that it forms a tetramer that contains a novel fold with a central, positively charged groove that binds to the viral RNA.
-
The viral non-structural protein NSs is the major virulence factor and antagonizes the host innate immune response by causing global inhibition of RNA polymerase II-mediated transcription.
-
Possession of a segmented genome enables orthobunyaviruses to evolve rapidly by segment reassortment during mixed infections. Reassortment occurs widely in nature and reassortant viruses can have dramatically altered properties, such as increased virulence.
-
Little is known about the burden of orthobunyavirus disease and there is a need for improved global surveillance to monitor orthobunyavirus activity.
Abstract
Orthobunyaviruses, which have small, tripartite, negative-sense RNA genomes and structurally simple virions composed of just four proteins, can have devastating effects on human health and well-being, either by causing disease in humans or by causing disease in livestock and crops. In this Review, I describe the recent genetic and structural advances that have revealed important insights into the composition of orthobunyavirus virions, viral transcription and replication and viral interactions with the host innate immune response. Lastly, I highlight outstanding questions and areas of future research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosenberg, R., Johansson, M. A., Powers, A. M. & Miller, B. R. Search strategy has influenced the discovery rate of human viruses. Proc. Natl Acad. Sci. USA 110, 13961–13964 (2013).
Vaheri, A. et al. Uncovering the mysteries of hantavirus infections. Nature Rev. Microbiol. 11, 539–550 (2013).
Calisher, C. H. in The Bunyaviridae (ed. Elliott, R. M.) 1–17 (Plenum Press, 1996).
Haddow, A. D. & Odoi, A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003–2007. PLoS ONE 4, e6145 (2009).
Beaty, B. & Calisher, C. Bunyaviridae — natural history. Curr. Top. Microbiol. Immunol. 169, 27–78 (1991).
Patrican, L. A. & DeFoliart, G. R. Lack of adverse effect of transovarially acquired La Crosse virus infection on the reproductive capacity of Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 22, 604–611 (1985).
Jackson, B. T., Brewster, C. C. & Paulson, S. L. La Crosse virus infection alters blood feeding behavior in Aedes triseriatus and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 49, 1424–1429 (2012).
Reese, S. M., Beaty, M. K., Gabitzsch, E. S., Blair, C. D. & Beaty, B. J. Aedes triseriatus females transovarially infected with La Crosse virus mate more efficiently than uninfected mosquitoes. J. Med. Entomol. 46, 1152–1158 (2009).
Thompson, W. H. & Beaty, B. J. Venereal transmission of La Crosse (California encephalitis) arbovirus in Aedes triseriatus mosquitoes. Science 196, 530–531 (1977).
Marklewitz, M. et al. Discovery of a unique novel clade of mosquito-associated bunyaviruses. J. Virol. 87, 12850–12865 (2013).
Taylor, K. G. & Peterson, K. E. Innate immune response to La Crosse virus infection. J. Neurovirol. 20, 150–156 (2014).
Bennett, R. S. et al. La Crosse virus infectivity, pathogenesis, and immunogenicity in mice and monkeys. Virol. J. 5, 25 (2008).
Varela, M. et al. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathog. 9, e1003133 (2013).
Wernike, K. et al. Schmallenberg virus experimental infection of sheep. Vet. Microbiol. 166, 461–466 (2013).
Barrett, A. D. T. & Shope, R. E. in Topley and Wilson's Microbiology and Microbial Infections. (eds Mahy, B. W. J. & Meulen, V.t.) 1025–1058 (Hodder Arnold, 2005).
Obijeski, J. F., Bishop, D. H., Murphy, F. A. & Palmer, E. L. Structural proteins of La Crosse virus. J. Virol. 19, 985–997 (1976).
Bowden, T. A. et al. Orthobunyavirus ultrastructure and the curious tripodal glycoprotein spike. PLoS Pathog. 9, e1003374 (2013). This cryoelectron microscopy study of Bunyamwera virus particles presents the three-dimensional structure of the glycoprotein spike resolved to 3 nm.
Battisti, A. J. et al. Structural studies of Hantaan virus. J. Virol. 85, 835–841 (2011).
Sherman, M. B., Freiberg, A. N., Holbrook, M. R. & Watowich, S. J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 387, 11–15 (2009).
Plyusnin, A. et al. in Virus taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. (eds King, A. M. Q., Adams, M. J., Carstens, E. B. & Lefkowits, E. J.) 725–741 (Elsevier, 2012).
Barr, J. N., Elliott, R. M., Dunn, E. F. & Wertz, G. W. Segment-specific terminal sequences of Bunyamwera bunyavirus regulate genome replication. Virology 311, 326–338 (2003).
Barr, J. N. & Wertz, G. W. Role of the conserved nucleotide mismatch within 3′- and 5′-terminal regions of Bunyamwera virus in signaling transcription. J. Virol. 79, 3586–3594 (2005).
Kohl, A., Dunn, E. F., Lowen, A. C. & Elliott, R. M. Complementarity, sequence and structural elements within the 3′ and 5′ non-coding regions of the Bunyamwera orthobunyavirus S segment determine promoter strength. J. Gen. Virol. 85, 3269–3278 (2004).
Kohl, A., Lowen, A. C., Leonard, V. H. J. & Elliott, R. M. Genetic elements regulating packaging of the Bunyamwera orthobunyavirus genome. J. Gen. Virol. 87, 177–187 (2006).
Osborne, J. C. & Elliott, R. M. RNA binding properties of bunyamwera virus nucleocapsid protein and selective binding to an element in the 5′ terminus of the negative-sense S segment. J. Virol. 74, 9946–9952 (2000).
Barr, J. N., Rodgers, J. W. & Wertz, G. W. Identification of the Bunyamwera bunyavirus transcription termination signal. J. Gen. Virol. 87, 189–198 (2006).
Blakqori, G., Lowen, A. C. & Elliott, R. M. The small genome segment of Bunyamwera orthobunyavirus harbours a single transcription termination signal. J. Gen. Virol. 93, 1449–1455 (2012).
Mazel-Sanchez, B. & Elliott, R. M. Attenuation of Bunyamwera orthobunyavirus replication by targeted mutagenesis of genomic UTRs and creation of viable viruses with minimal genome segments. J. Virol. 86, 13672–13678 (2012).
Elliott, R. M. & Blakqori, G. in Bunyaviridae. Molecular and Cellular Biology (eds Plyusnin, A. & Elliott, R. M.) 1–39 (Caister Academic Press, 2011).
Fazakerley, J. K. et al. Organization of the middle RNA segment of snowshoe hare Bunyavirus. Virology 167, 422–432 (1988).
Fuller, F., Bhown, A. S. & Bishop, D. H. Bunyavirus nucleoprotein, N, and a non-structural protein, NSS, are coded by overlapping reading frames in the S RNA. J. Gen. Virol. 64, 1705–1714 (1983).
Mohamed, M., McLees, A. & Elliott, R. M. Viruses in the Anopheles A, Anopheles B, and Tete serogroups in the Orthobunyavirus genus (family Bunyaviridae) do not encode an NSs protein. J. Virol. 83, 7612–7618 (2009).
Chowdhary, R. et al. Genetic characterization of the Wyeomyia group of orthobunyaviruses and their phylogenetic relationships. J. Gen. Virol. 93, 1023–1034 (2012).
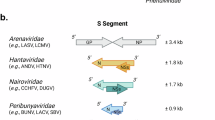
Lanciotti, R. S. et al. Isolation of a novel orthobunyavirus (Brazoran virus) with a 1.7 kb S segment that encodes a unique nucleocapsid protein possessing two putative functional domains. Virology 444, 55–63 (2013).
Jin, H. & Elliott, R. M. Mutagenesis of the L protein encoded by Bunyamwera virus and production of monoclonal antibodies. J. Gen. Virol. 73, 2235–2244 (1992).
Reguera, J., Weber, F. & Cusack, S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 6, e1001101 (2010). This paper characterizes the endonuclease domain that is located in the N terminus of the orthobunyavirus RdRp, which generates the capped primers that are required for viral mRNA synthesis.
Patterson, J. L., Holloway, B. & Kolakofsky, D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J. Virol. 52, 215–222 (1984).
Rossier, C., Patterson, J. & Kolakofsky, D. La Crosse virus small genome mRNA is made in the cytoplasm. J. Virol. 58, 647–650 (1986).
Shi, X., Lappin, D. F. & Elliott, R. M. Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins. J. Virol. 78, 10793–10802 (2004). This study shows that the Golgi-targeting signal is present in the transmembrane domain of the orthobunyavirus Gn protein.
Garry, C. E. & Garry, R. F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of Bunyaviruses are class II viral fusion protein (β-penetrenes). Theor. Biol. Med. Model. 1, 10 (2004).
Plassmeyer, M. L. et al. Mutagenesis of the La Crosse virus glycoprotein supports a role for Gc (1066–1087) as the fusion peptide. Virology 358, 273–282 (2007).
Shi, X., Goli, J., Clark, G., Brauburger, K. & Elliott, R. M. Functional analysis of the Bunyamwera orthobunyavirus Gc glycoprotein. J. Gen. Virol. 90, 2483–2492 (2009).
Shi, X., van Mierlo, J. T., French, A. & Elliott, R. M. Visualizing the replication cycle of bunyamwera orthobunyavirus expressing fluorescent protein-tagged Gc glycoprotein. J. Virol. 84, 8460–8469 (2010). This paper describes the generation of a recombinant BUNV in which the Gc glycoprotein is fused to GFP, which enables viral propagation to be monitored by light microscopy.
Ogg, M. M. & Patterson, J. L. RNA binding domain of Jamestown Canyon virus S segment RNAs. J. Virol. 81, 13754–13760 (2007).
Shi, X. et al. Requirement of the N-terminal region of orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 80, 8089–8099 (2006).
Dong, H., Li, P., Bottcher, B., Elliott, R. M. & Dong, C. Crystal structure of Schmallenberg orthobunyavirus nucleoprotein–RNA complex reveals a novel RNA sequestration mechanism. RNA 19, 1129–1136 (2013).
Dong, H., Li, P., Elliott, R. M. & Dong, C. Structure of Schmallenberg orthobunyavirus nucleoprotein suggests a novel mechanism of genome encapsidation. J. Virol. 87, 5593–5601 (2013).
Ariza, A. et al. Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization. Nucleic Acids Res. 41, 5912–5926 (2013).
Li, B. et al. Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc. Natl Acad. Sci. USA 110, 9048–9053 (2013).
Niu, F. et al. Structure of the Leanyer orthobunyavirus nucleoprotein–RNA complex reveals unique architecture for RNA encapsidation. Proc. Natl Acad. Sci. USA 110, 9054–9059 (2013).
Reguera, J., Malet, H., Weber, F. & Cusack, S. Structural basis for encapsidation of genomic RNA by La Crosse orthobunyavirus nucleoprotein. Proc. Natl Acad. Sci. USA 110, 7246–7251 (2013). References 47, 48, 49, 50 and 51 report crystal structures of orthobunyavirus nucleocapsid proteins.
Zheng, W. & Tao, Y. J. Genome encapsidation by orthobunyavirus nucleoproteins. Proc. Natl Acad. Sci. USA 110, 8769–8770 (2013).
Leonard, V. H., Kohl, A., Osborne, J. C., McLees, A. & Elliott, R. M. Homotypic interaction of Bunyamwera virus nucleocapsid protein. J. Virol. 79, 13166–13172 (2005).
Eifan, S. A. & Elliott, R. M. Mutational analysis of the Bunyamwera orthobunyavirus nucleocapsid protein gene. J. Virol. 83, 11307–11317 (2009).
Nakitare, G. W. & Elliott, R. M. Expression of the Bunyamwera virus M genome segment and intracellular localisation of NSm. Virology 195, 511–520 (1993).
Lappin, D. F., Nakitare, G. W., Palfreyman, J. W. & Elliott, R. M. Localisation of Bunyamwera bunyavirus G1 glycoprotein to the Golgi requires association with G2 but not NSm. J. Gen. Virol. 75, 3441–3451 (1994).
Fontana, J., Lopez-Montero, N., Elliott, R. M., Fernandez, J. J. & Risco, C. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell. Microbiol. 10, 2012–2028 (2008).
Bupp, K., Stillmock, K. & Gonzalez-Scarano, F. Analysis of the intracellular transport properties of recombinant La Crosse virus glycoproteins. Virology 220, 485–490 (1996).
Thomas, D. et al. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 279, 31471–31477 (2004).
Eifan, S., Schnettler, E., Dietrich, I., Kohl, A. & Blomstrom, A. L. Non-structural proteins of arthropod-borne bunyaviruses: roles and functions. Viruses 5, 2447–2468 (2013).
Bridgen, A., Weber, F., Fazakerley, J. K. & Elliott, R. M. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl Acad. Sci. USA 98, 664–669 (2001).
Blakqori, G. & Weber, F. Efficient cDNA-based rescue of La Crosse bunyaviruses expressing or lacking the nonstructural protein NSs. J. Virol. 79, 10420–10428 (2005).
Elliott, R. M. et al. Establishment of a reverse genetics system for Schmallenberg virus, a newly emerged orthobunyavirus in Europe. J. Gen. Virol. 94, 851–859 (2013).
Hart, T. J., Kohl, A. & Elliott, R. M. Role of the NSs protein in the zoonotic capacity of Orthobunyaviruses. Zoonoses Publ. Health 56, 285–296 (2009).
Kohl, A. et al. Bunyamwera virus nonstructural protein NSs counteracts interferon regulatory factor 3-mediated induction of early cell death. J. Virol. 77, 7999–8008 (2003).
Mukherjee, P., Woods, T. A., Moore, R. A. & Peterson, K. E. Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity 38, 705–716 (2013).
Colon-Ramos, D. A. et al. Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper. Mol. Biol. Cell 14, 4162–4172 (2003).
Weber, F., Dunn, E. F., Bridgen, A. & Elliott, R. M. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281, 67–74 (2001).
Blakqori, G., Kochs, G., Haller, O. & Weber, F. Functional L polymerase of La Crosse virus allows in vivo reconstitution of recombinant nucleocapsids. J. Gen. Virol. 84, 1207–1214 (2003).
Lozach, P. Y. et al. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10, 75–88 (2011).
Santos, R. I. et al. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 138, 139–143 (2008).
Hollidge, B. S. et al. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 86, 7988–8001 (2012). This paper describes the entry mechanism of LACV into cells of the central nervous system.
Hacker, J. K. & Hardy, J. L. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology 235, 40–47 (1997).
Ludwig, G. V., Christensen, B. M., Yuill, T. M. & Schultz, K. T. Enzyme processing of La Crosse virus glycoprotein G1: a bunyavirus-vector infection model. Virology 171, 108–113 (1989).
Ludwig, G. V., Israel, B. A., Christensen, B. M., Yuill, T. M. & Schultz, K. T. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology 181, 564–571 (1991).
Jin, H. & Elliott, R. M. Characterisation of Bunyamwera virus S RNA that is transcribed and replicated by the L protein expressed from recombinant vaccinia virus. J. Virol. 67, 1396–1404 (1993).
Blakqori, G., van Knippenberg, I. & Elliott, R. M. Bunyamwera orthobunyavirus S-segment untranslated regions mediate poly(A) tail-independent translation. J. Virol. 83, 3637–3646 (2009).
Kolakofsky, D., Bellocq, C. & Raju, R. The translational requirement for La Crosse virus S-mRNA synthesis. Cold Spring Harb. Symp. Quant. Biol. 52, 373–379 (1987).
Patterson, J. L. & Kolakofsky, D. Characterization of La Crosse virus small-genome transcripts. J. Virol. 49, 680–685 (1984).
Vialat, P. & Bouloy, M. Germiston virus transcriptase requires active 40S ribosomal subunits and utilizes capped cellular RNAs. J. Virol. 66, 685–693 (1992).
Bellocq, C., Raju, R., Patterson, J. & Kolakofsky, D. Translational requirement of La Crosse virus S-mRNA synthesis: in vitro studies. J. Virol. 61, 87–95 (1987).
Raju, R. & Kolakofsky, D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J. Virol. 61, 96–103 (1987).
Barr, J. N. Bunyavirus mRNA synthesis is coupled to translation to prevent premature transcription termination. RNA 13, 731–736 (2007). This study explores the unique requirement for ongoing protein synthesis for orthobunyavirus mRNA synthesis and demonstrates that the translation of nascent mRNAs prevents premature termination of viral transcription.
Dunn, E. F., Pritlove, D. C., Jin, H. & Elliott, R. M. Transcription of a recombinant bunyavirus RNA template by transiently expressed bunyavirus proteins. Virology 211, 133–143 (1995).
Shi, X., Kohl, A., Li, P. & Elliott, R. M. Role of the cytoplasmic tail domains of Bunyamwera orthobunyavirus glycoproteins Gn and Gc in virus assembly and morphogenesis. J. Virol. 81, 10151–10160 (2007).
Salanueva, I. J. et al. Polymorphism and structural maturation of Bunyamwera virus in Golgi and post-Golgi compartments. J. Virol. 77, 1368–1381 (2003).
Novoa, R. R., Calderita, G., Cabezas, P., Elliott, R. M. & Risco, C. Key Golgi factors for structural and functional maturation of bunyamwera virus. J. Virol. 79, 10852–10863 (2005).
Madoff, D. H. & Lenard, J. A membrane glycoprotein that accumulates intracellularly: cellular processing of the large glycoprotein of LaCrosse virus. Cell 28, 821–829 (1982).
Hutchinson, E. C., von Kirchbach, J. C., Gog, J. R. & Digard, P. Genome packaging in influenza A virus. J. Gen. Virol. 91, 313–328 (2010).
Lowen, A. C., Boyd, A., Fazakerley, J. K. & Elliott, R. M. Attenuation of bunyavirus replication by rearrangement of viral coding and noncoding sequences. J. Virol. 79, 6940–6946 (2005).
Sanz-Sanchez, L. & Risco, C. Multilamellar structures and filament bundles are found on the cell surface during bunyavirus egress. PLoS ONE 8, e65526 (2013).
Borucki, M. K., Kempf, B. J., Blitvich, B. J., Blair, C. D. & Beaty, B. J. La Crosse virus: replication in vertebrate and invertebrate hosts. Microbes Infect. 4, 341–350 (2002).
Scallan, M. F. & Elliott, R. M. Defective RNAs in mosquito cells persistently infected with Bunyamwera virus. J. Gen. Virol. 73, 53–60 (1992).
Hacker, D., Raju, R. & Kolakofsky, D. La Crosse virus nucleocapsid protein controls its own synthesis in mosquito cells by encapsidating its mRNA. J. Virol. 63, 5166–5174 (1989). This study shows that the dsRNA panhandle structure in LACV nucleocapsids triggers the innate immune response by activating RIGI shortly after viral entry.
Szemiel, A. M., Failloux, A. B. & Elliott, R. M. Role of Bunyamwera orthobunyavirus NSs protein in infection of mosquito cells. PLoS Negl Trop. Dis. 6, e1823 (2012).
Lopez-Montero, N. & Risco, C. Self-protection and survival of arbovirus-infected mosquito cells. Cell. Microbiol. 13, 300–315 (2011).
Randall, R. E. & Goodbourn, S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89, 1–47 (2008).
Schoggins, J. W. & Rice, C. M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 1, 519–525 (2011).
Weber, M. et al. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe 13, 336–346 (2013).
Kochs, G., Janzen, C., Hohenberg, H. & Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl Acad. Sci. USA 99, 3153–3158 (2002).
Streitenfeld, H. et al. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 77, 5507–5511 (2003).
Carlton-Smith, C. & Elliott, R. M. Viperin, MTAP44, and protein kinase R contribute to the interferon-induced inhibition of Bunyamwera orthobunyavirus replication. J. Virol. 86, 11548–11557 (2012).
Schoggins, J. W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695 (2014).
Weber, F. et al. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76, 7949–7955 (2002).
Blakqori, G. et al. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J. Virol. 81, 4991–4999 (2007).
Leonard, V. H., Kohl, A., Hart, T. J. & Elliott, R. M. Interaction of Bunyamwera Orthobunyavirus NSs protein with mediator protein MED8: a mechanism for inhibiting the interferon response. J. Virol. 80, 9667–9675 (2006).
Poss, Z. C., Ebmeier, C. C. & Taatjes, D. J. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 48, 575–608 (2013).
van Knippenberg, I., Carlton-Smith, C. & Elliott, R. M. The N-terminus of Bunyamwera orthobunyavirus NSs protein is essential for interferon antagonism. J. Gen. Virol. 91, 2002–2006 (2010).
Verbruggen, P. et al. Interferon antagonist NSs of La Crosse virus triggers a DNA damage response-like degradation of transcribing RNA polymerase II. J. Biol. Chem. 286, 3681–3692 (2011). This paper describes the mechanism by which the LACV NSs protein degrades host cell RNA polymerase II to evade the innate immune response of the host.
Briese, T., Calisher, C. H. & Higgs, S. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology 446, 207–216 (2013). This provocative paper discusses the importance of reassortment in bunyavirus evolution.
Wernike, K. et al. Schmallenberg virus — two years of experiences. Prev. Vet. Med. http://dx.doi.org/10.1016/j.prevetmed.2014.03.021 (2014).
Kim, Y. H. et al. Development of inactivated trivalent vaccine for the teratogenic Aino, Akabane and Chuzan viruses. Biologicals 39, 152–157 (2011).
Wernike, K., Nikolin, V. M., Hechinger, S., Hoffmann, B. & Beer, M. Inactivated Schmallenberg virus prototype vaccines. Vaccine 31, 3558–3563 (2013).
Donald, C. L., Kohl, A. & Schnettler, E. New insights into control of arbovirus replication and spread by insect RNA interference pathways. Insects 3, 511–531 (2012).
Hoffmann, B. et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 18, 469–472 (2012). This paper describes the initial characterization of Schmallenberg virus as a new orthobunyavirus and provides the first report of a Simbu serogroup virus in Europe.
van den Brom, R. et al. Epizootic of ovine congenital malformations associated with Schmallenberg virus infection. Tijdschr Diergeneeskd 137, 106–111 (2012).
Afonso, A. et al. The Schmallenberg virus epidemic in Europe — 2011–2013. Prev. Vet. Med. http://dx.doi.org/10.1016/j.prevetmed.2014.02.012 (2014).
European Commission. Statement on the Schmallenberg Virus Situation issued by the European Commission together with the EU Member States following the working group held on 17 February 2012. [online] (2012).
Harris, K. A. et al. The impact of Schmallenberg virus on British sheep farms during the 2011/2012 lambing season. Vet. Rec. http://dx.doi.org/10.1136/vr.102295 (2014).
Goller, K. V., Hoper, D., Schirrmeier, H., Mettenleiter, T. C. & Beer, M. Schmallenberg virus as possible ancestor of Shamonda virus. Emerg. Infect. Dis. 18, 1644–1646 (2012).
Veronesi, E. et al. Implicating Culicoides biting midges as vectors of Schmallenberg virus using semi-quantitative RT-PCR. PLoS ONE 8, e57747 (2013).
Sedda, L. & Rogers, D. J. The influence of the wind in the Schmallenberg virus outbreak in Europe. Sci. Rep. 3, 3361 (2013). This study models the contribution of wind to the spread of Schmallenberg virus and suggests that most infections originate from the bite of midges that are spread by downwind movements.
Bilk, S. et al. Organ distribution of Schmallenberg virus RNA in malformed newborns. Vet. Microbiol. 159, 236–238 (2012).
Kupferschmidt, K. Scientists rush to find clues on new animal virus. Science 335, 1028–1029 (2012).
Larska, M., Lechowski, L., Grochowska, M. & Zmudzinski, J. F. Detection of the Schmallenberg virus in nulliparous Culicoides obsoletus/scoticus complex and C. punctatus — the possibility of transovarial virus transmission in the midge population and of a new vector. Vet. Microbiol. 166, 467–473 (2013).
Meroc, E. et al. Follow-up of the Schmallenberg virus seroprevalence in Belgian cattle. Transbound. Emerg. Dis. http://dx.doi.org/10.1111/tbed.12202 (2013).
Hulst, M. et al. Genetic characterization of an atypical Schmallenberg virus isolated from the brain of a malformed lamb. Virus Genes 47, 505–514 (2013).
Rosseel, T. et al. DNase SISPA-next generation sequencing confirms Schmallenberg virus in Belgian field samples and identifies genetic variation in Europe. PLoS ONE 7, e41967 (2012).
Coupeau, D., Claine, F., Wiggers, L., Kirschvink, N. & Muylkens, B. In vivo and in vitro identification of a hypervariable region in Schmallenberg virus. J. Gen. Virol. 94, 1168–1174 (2013).
Fischer, M. et al. A mutation 'hot spot' in the Schmallenberg virus M segment. J. Gen. Virol. 94, 1161–1167 (2013).
Bridgen, A. & Elliott, R. M. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl Acad. Sci. USA 93, 15400–15404 (1996). This paper describes the initial reverse genetics system for Bunyamwera virus, which was the first system used to generate a negative-sense RNA virus with a segmented genome from plasmid DNAs.
Lowen, A. C., Noonan, C., McLees, A. & Elliott, R. M. Efficient bunyavirus rescue from cloned cDNA. Virology 330, 493–500 (2004).
Ogawa, Y., Sugiura, K., Kato, K., Tohya, Y. & Akashi, H. Rescue of Akabane virus (family Bunyaviridae) entirely from cloned cDNAs by using RNA polymerase I. J. Gen. Virol. 88, 3385–3390 (2007).
Iroegbu, C. U. & Pringle, C. R. Genetic interactions among viruses of the Bunyamwera complex. J. Virol. 37, 383–394 (1981).
Urquidi, V. & Bishop, D. H. Non-random reassortment between the tripartite RNA genomes of La Crosse and snowshoe hare viruses. J. Gen. Virol. 73, 2255–2265 (1992).
Beaty, B. J., Bishop, D. H., Gay, M. & Fuller, F. Interference between bunyaviruses in Aedes triseriatus mosquitoes. Virology 127, 83–90 (1983).
Beaty, B. J. et al. Molecular basis of bunyavirus transmission by mosquitoes: role of the middle-sized RNA segment. Science 211, 1433–1435 (1981).
Briese, T., Bird, B., Kapoor, V., Nichol, S. T. & Lipkin, W. I. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J. Virol. 80, 5627–5630 (2006).
Acknowledgements
Work in the author's laboratory is supported by a Wellcome Trust Senior Investigator Award. The author apologizes to colleagues whose work could not be cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Arthrogryposis
-
A congenital abnormality in which the limb joints are deformed and inflexible.
- Hydranencephaly
-
A condition that arises during foetal development, in which the central cavity of the brain is missing and is replaced with cerebrospinal fluid.
- Complement fixation
-
An immunological test to measure whether antibody–antigen complexes can deplete a known concentration of standard complement proteins and protect added red blood cells from lysis. For orthobunyaviruses, complement-fixing antibodies are elicited by the nucleocapsid protein and are used to define serogroups.
- Neutralization
-
An immunological test to detect antibodies that bind to a virus and reduce its titre in an infectivity assay. For orthobunyaviruses, neutralizing antibodies are elicited by the viral glycoproteins and are used for the identification of specific viruses.
- Haemagglutination inhibition
-
An immunological test to measure the ability of antibodies to block the agglutination of red blood cells by a virus. For orthobunyaviruses, the viral glycoproteins mediate haemagglutination, and antibodies to these proteins that inhibit haemagglutination are used to define serogroups.
- Cryoelectron microscopy
-
A technique that preserves the native state of biological material in a frozen hydrated state, which is usually achieved by rapid freezing in liquid ethane. Specimens are examined in a transmission electron microscope at ultralow temperatures and can be visualized without the need for heavy-metal staining.
- Subtomographic averaging
-
An electron microscopy technique to visualize structures in three dimensions (3D). The specimen stage is tilted at regular increments and a series of transmission electron images is taken, which are then processed to generate a high-resolution (up to 5 nm) 3D image.
- Leaky ribosomal scanning
-
A mechanism by which internal AUG codons in an mRNA are used to initiate translation. Ribosomes occasionally skip the first AUG codon, which is often not in an optimal sequence context for initiation, and a downstream AUG is selected instead.
- Cap snatching
-
A mechanism to prime viral mRNA synthesis. A viral endonuclease cleaves the 5′ ends of cellular mRNAs about 12–18 nucleotides after the 5′ 7-methylguanylate residue (the cap), and the resulting short oligonucleotides are used to initiate viral mRNA transcription.
- Class II fusion domain
-
Proteins that contain this domain mediate the fusion of viral membranes to cellular membranes. Class II fusion peptides, which are also known as betapenetrenes, are found internally in the surface glycoproteins of bunyaviruses, alphaviruses and flaviviruses and mostly comprise of β-structures.
- Minigenome
-
A genome analogue that comprises the 3′ and 5′ UTRs of a viral genome segment and contains a reporter gene (for example, luciferase) in the negative-sense in place of the viral coding sequence.
- DC-SIGN
-
(Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin). A calcium-dependent carbohydrate-binding protein (C-type lectin) receptor that is present on the surface of both macrophages and dendritic cells.
- Macropinocytosis
-
A process that involves the uptake of molecules into cells in a clathrin-independent manner. Membrane ruffles trap fluid as they fold over themselves at the cell surface and undergo fusion, which results in vesicles that pinch off the membrane surface and hence deliver cargo to the cytoplasm.
- Caveolar endocytosis
-
A clathrin-independent mechanism of endocytosis. Entry into cells is mediated by caveolae, which are flask-shaped invaginations on the cell surface that contain the protein caveolin.
- Rabbit reticulocyte lysate
-
An in vitro or cell-free translation system that is based on nuclease-treated lysates of reticulocytes (the cells that are responsible for the production of haemoglobin), which enables the translation of exogenous RNAs.
- Virus factory
-
A region of the host cell that is modified by the virus, such that it recruits cellular organelles and functions as a scaffold for virus assembly.
- Endoglycosidase H
-
(Endo H). An enzyme that cleaves asparagine-linked high-mannose glycans from glycoproteins but does not digest complex oligosaccharide side chains. EndoH sensitivity is used as a marker to monitor oligosaccharide processing of a protein as it transits through the Golgi complex.
- Exocytic pathway
-
The exocytic pathway consists of a series of membrane-bound organelles that transport proteins and lipids in vesicles from the ER to the cell surface.
- RNAi
-
In the context of this Review, a gene-silencing mechanism in which viral RNAs are targeted for destruction by specific small interfering RNA molecules.
- Pattern recognition receptors
-
(PRRs). A diverse group of membrane and cytoplasmic proteins that identify various microbial pathogen components to trigger the innate immune system.
- Mediator
-
A multiprotein complex that functions as a key component of the RNA polymerase II transcriptional machinery. It functions as a bridge between the enzyme and regulatory proteins and is involved in both activation and repression of mRNA production.
Rights and permissions
About this article
Cite this article
Elliott, R. Orthobunyaviruses: recent genetic and structural insights. Nat Rev Microbiol 12, 673–685 (2014). https://doi.org/10.1038/nrmicro3332
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro3332
This article is cited by
-
Evaluation of the vector competence for Batai virus of native Culex and exotic Aedes species in Central Europe
Parasites & Vectors (2024)
-
Differential role of NSs genes in the neurovirulence of two genogroups of Akabane virus causing postnatal encephalomyelitis
Archives of Virology (2024)
-
Glucosylceramide in bunyavirus particles is essential for virus binding to host cells
Cellular and Molecular Life Sciences (2024)
-
Vaccine development against Schmallenberg virus: from classical inactivated to modified-live to scaffold particle vaccines
One Health Outlook (2022)
-
Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development
npj Vaccines (2022)