Key Points
-
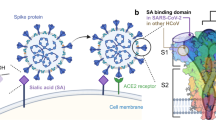
Many viruses engage sialylated glycans to bind to and infect cells. Sialic acid-binding viruses include influenza virus, reovirus, adenovirus and rotavirus, which bind to host receptors via their stalk-like attachment proteins.
-
Glycan microarray technology has enabled the rapid identification of specific carbohydrate ligands for several viruses. This technology has been used to discern glycan-binding preferences between closely related strains, including pathogenic influenza virus isolates.
-
Improvements in X-ray crystallography have enabled the virus–glycan interaction to be studied in detail and, coupled with reverse genetics approaches, structure–function relationships of virus–glycan interactions can be determined in vitro and in vivo.
-
The complexity of virus–sialic acid interactions is remarkable, as linkage basis and sialic acid modifications, such as sulphation, influence binding capacity. Preferences in glycan engagement influence influenza virus host range and modulate tissue tropism.
-
Virus–sialylated glycan interactions are an important therapeutic target, and structure–function analysis has facilitated drug development and may improve oncolytic vector design.
Abstract
Viral infections are initiated by attachment of the virus to host cell surface receptors, including sialic acid-containing glycans. It is now possible to rapidly identify specific glycan receptors using glycan array screening, to define atomic-level structures of virus–glycan complexes and to alter the glycan-binding site to determine the function of glycan engagement in viral disease. This Review highlights general principles of virus–glycan interactions and provides specific examples of sialic acid binding by viruses with stalk-like attachment proteins, including influenza virus, reovirus, adenovirus and rotavirus. Understanding virus–glycan interactions is essential to combating viral infections and designing improved viral vectors for therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Barton, E. S., Connolly, J. L., Forrest, J. C., Chappell, J. D. & Dermody, T. S. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276, 2200–2211 (2001).
Chappell, J. D., Duong, J. L., Wright, B. W. & Dermody, T. S. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74, 8472–8479 (2000).
Chappell, J. D., Gunn, V. L., Wetzel, J. D., Baer, G. S. & Dermody, T. S. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein s1. J. Virol. 71, 1834–1841 (1997).
Tsai, B. et al. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22, 4346–4355 (2003).
Rogers, G. N. et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304, 76–78 (1983).
Neu, U., Bauer, J. & Stehle, T. Viruses and sialic acids: rules of engagement. Curr. Opin. Struct. Biol. 21, 610–618 (2011).
Silva, L. A. et al. A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. J. Virol. 88, 2385–2397 (2014).
Gardner, C. L. et al. Natural variation in the heparan sulfate binding domain of the eastern equine encephalitis virus E2 glycoprotein alters interactions with cell surfaces and virulence in mice. J. Virol. 87, 8582–8590 (2013).
Gardner, C. L., Ebel, G. D., Ryman, K. D. & Klimstra, W. B. Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc. Natl Acad. Sci. USA 108, 16026–16031 (2011).
Tiwari, V., Maus, E., Sigar, I. M., Ramsey, K. H. & Shukla, D. Role of heparan sulfate in sexually transmitted infections. Glycobiology 22, 1402–1412 (2012).
Hu, L. et al. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 485, 256–259 (2012). This study identifies HBGA as a receptor for human rotavirus strain HAL1166 and defines the glycan-binding site using X-ray crystallography. Interestingly, only modest changes in the glycan-binding site greatly alter glycan-binding specificity among rotavirus strains.
Liu, Y. et al. Poly-LacNAc as an age-specific ligand for rotavirus P[11] in neonates and infants. PLoS ONE 8, e78113 (2013).
Neu, U. et al. Structures of Merkel cell polyomavirus VP1 complexes define a sialic acid binding site required for infection. PLoS Pathog. 8, e1002738 (2012).
Neu, U. et al. Structure–function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe 8, 309–319 (2010).
Varki, A. Multiple changes in sialic acid biology during human evolution. Glycoconjugate J. 26, 231–245 (2009). This review provides insights into the host–pathogen 'arms race' throughout the course of human evolution.
Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446, 1023–1029 (2007).
Schwarzkopf, M. et al. Sialylation is essential for early development in mice. Proc. Natl Acad. Sci. USA 99, 5267–5270 (2002).
Svennerholm, L. Interaction of cholera toxin and ganglioside G(M1). Adv. Exp. Med. Biol. 71, 191–204 (1976).
Reiss, K. et al. The GM2 glycan serves as a functional co-receptor for serotype 1 reovirus. PLoS Pathog. 8, e1003078 (2012).
Rogers, G. N., Pritchett, T. J., Lane, J. L. & Paulson, J. C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology 131, 394–408 (1983).
Nilsson, E. C. et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nature Med. 17, 105–109 (2011). This study identifies GD1a as a cellular receptor for Ad37 and defines the glycan-binding site using NMR spectroscopy and X-ray crystallography.
Miller-Podraza, H., Bradley, R. M. & Fishman, P. H. Biosynthesis and localization of gangliosides in cultured cells. Biochemistry 21, 3260–3265 (1982).
Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl Acad. Sci. USA 101, 17033–17038 (2004).
Feizi, T., Fazio, F., Chai, W. & Wong, C. H. Carbohydrate microarrays — a new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 13, 637–645 (2003).
Liu, Y. et al. Neoglycolipid-based oligosaccharide microarray system: preparation of NGLs and their noncovalent immobilization on nitrocellulose-coated glass slides for microarray analyses. Methods Mol. Biol. 808, 117–136 (2012).
Liu, Y., Palma, A. S. & Feizi, T. Carbohydrate microarrays: key developments in glycobiology. Biol. Chem. 390, 647–656 (2009). This review provides a comprehensive description of glycan array technology.
Childs, R. A. et al. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nature Biotech. 27, 797–799 (2009). This study uses glycan array technology to determine the glycan-binding specificity of pandemic H1N1 in comparison to the 2009 seasonal strain. It is an example of how glycan arrays can be used to identify specific glycan receptors for emerging virus strains.
Stevens, J. et al. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 355, 1143–1155 (2006). This study is an important example of how glycan array technology can be used to determine differences in receptor specificity between influenza virus strains.
Ramani, S. et al. The VP8* domain of neonatal rotavirus strain G10P[11] binds to type II precursor glycans. J. Virol. 87, 7255–7264 (2013). This study defines the glycan receptor specificity of neonatal rotavirus infections.
Neu, U., Woellner, K., Gauglitz, G. & Stehle, T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proc. Natl Acad. Sci. USA 105, 5219–5224 (2008).
Xu, R. et al. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science 342, 1230–1235 (2013).
Gulati, S., Lasanajak, Y., Smith, D. F., Cummings, R. D. & Air, G. M. Glycan array analysis of influenza H1N1 binding and release. Cancer Biomark. 14, 43–53 (2014).
Fukui, S., Feizi, T., Galustian, C., Lawson, A. M. & Chai, W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate–protein interactions. Nature Biotech. 20, 1011–1017 (2002).
Palma, A. S., Feizi, T., Childs, R. A., Chai, W. & Liu, Y. The neoglycolipid (NGL)-based oligosaccharide microarray system poised to decipher the meta-glycome. Curr. Opin. Chem. Biol. 18, 1–8 (2014).
Wang, L. et al. Cross-platform comparison of glycan microarray formats. Glycobiology 24, 507–517 (2014).
Padler-Karavani, V. et al. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J. Biol. Chem. 287, 22593–22608 (2012).
Engelhardt, O. G. Many ways to make an influenza virus — review of influenza virus reverse genetics methods. Influenza Other Respir. Viruses 7, 249–256 (2013).
Fodor, E. et al. Rescue of influenza A virus from recombinant DNA. J. Virol. 73, 9679–9682 (1999).
Boehme, K. W., Ikizler, M., Kobayashi, T. & Dermody, T. S. Reverse genetics for mammalian reovirus. Methods 55, 109–113 (2011). This study describes methods for mammalian reovirus reverse genetics in detail.
Kobayashi, T., Ooms, L. S., Ikizler, M., Chappell, J. D. & Dermody, T. S. An improved reverse genetics system for mammalian orthoreoviruses. Virology 2, 194–200 (2010).
Adams, P. D. et al. Advances, interactions, and future developments in the CNS, Phenix, and Rosetta structural biology software systems. Annu. Rev. Biophys. 42, 265–287 (2013).
Wlodawer, A., Minor, W., Dauter, Z. & Jaskolski, M. Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination. FEBS J. 280, 5705–5736 (2013).
Rademacher, C., Krishna, N. R., Palcic, M., Parra, F. & Peters, T. NMR experiments reveal the molecular basis of receptor recognition by a calicivirus. J. Am. Chem. Soc. 130, 3669–3675 (2008).
Weis, W. et al. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333, 426–431 (1988).
Nobusawa, E. et al. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182, 475–485 (1991).
Watowich, S. J., Skehel, J. J. & Wiley, D. C. Crystal structures of influenza virus hemagglutinin in complex with high-affinity receptor analogs. Structure 2, 719–731 (1994).
Sauter, N. K. et al. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 31, 9609–9621 (1992).
Xiong, X. et al. Receptor binding by an H7N9 influenza virus from humans. Nature 499, 496–499 (2013).
Xiong, X. et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497, 392–396 (2013).
Zhang, Y. et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 340, 1459–1463 (2013).
Chandrasekaran, A. et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nature Biotech. 26, 107–113 (2008).
Matrosovich, M. et al. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74, 8502–8512 (2000).
Connor, R. J., Kawaoka, Y., Webster, R. G. & Paulson, J. C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205, 17–23 (1994).
Imai, M. & Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2, 160–167 (2012).
Stevens, J., Blixt, O., Paulson, J. C. & Wilson, I. A. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nature Rev. Microbiol. 4, 857–864 (2006).
Glaser, L. et al. A single amino acid substitution in 1918 influenza virus hemagglutinin changes receptor binding specificity. J. Virol. 79, 11533–11536 (2005).
Liu, Y. et al. Altered receptor specificity and cell tropism of D222G hemagglutinin mutants isolated from fatal cases of pandemic A(H1N1) 2009 influenza virus. J. Virol. 84, 12069–12074 (2010).
Bradley, K. C. et al. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (Novel 2009 H1N1). Virology 413, 169–182 (2011).
Gulati, S. et al. Human H3N2 influenza viruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS ONE 8, e66325 (2013). This study reveals the remarkable diversity in influenza virus haemagglutinin binding preferences using glycan array screening.
Crusat, M. et al. Changes in the hemagglutinin of H5N1 viruses during human infection — influence on receptor binding. Virology 447, 326–337 (2013).
Belser, J. A., Katz, J. M. & Tumpey, T. M. The ferret as a model organism to study influenza A virus infection. Dis. Models Mechanisms 4, 575–579 (2011).
Imai, M. et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 (2012).
Linster, M. et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157, 329–339 (2014). This study identifies the precise genetic changes that are required for influenza virus transmissibility.
de Graaf, M. & Fouchier, R. A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 33, 823–841 (2014). This study reviews the function of receptor binding in influenza virus pathogenesis in detail.
Tai, J. H. et al. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J. Infect. Dis. 191, 1221–1224 (2005).
Reiter, D. M. et al. Crystal structure of reovirus attachment protein σ1 in complex with sialylated oligosaccharides. PLoS Pathog. 7, e1002166 (2011).
Kirchner, E., Guglielmi, K. M., Strauss, H. M., Dermody, T. S. & Stehle, T. Structure of reovirus s1 in complex with its receptor junctional adhesion molecule-A. PLoS Pathog. 4, e1000235 (2008).
Barton, E. S. et al. Junction adhesion molecule is a receptor for reovirus. Cell 104, 441–451 (2001).
Antar, A. A. R. et al. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5, 59–71 (2009).
Bazzoni, G. et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275, 20520–20526 (2000).
Ebnet, K., Schulz, C. U., Meyer Zu Brickwedde, M. K., Pendl, G. G. & Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275, 27979–27988 (2000).
Maschio, A. D. et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhabited by an antibody to junctional adhesion molecule (JAM). J. Exp. Med. 190, 1351–1356 (1999).
Lechner, F. et al. Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J. Infect. Dis. 182, 978–982 (2000).
Lerner, A. M., Cherry, J. D. & Finland, M. Haemagglutination with reoviruses. Virology 19, 58–65 (1963).
Fukuda, M., Lauffenburger, M., Sasaki, H., Rogers, M. E. & Dell, A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J. Biol. Chem. 262, 11952–11957 (1987).
Pahlsson, P., Blackall, D. P., Ugorski, M., Czerwinski, M. & Spitalnik, S. L. Biochemical characterization of the O-glycans on recombinant glycophorin A expressed in Chinese hamster ovary cells. Glycoconjugate J. 11, 43–50 (1994).
Eggers, H. J., Gomatos, P. J. & Tamm, I. Agglutination of bovine erythrocytes: a general characteristic of reovirus type 3. Proc. Soc. Exp. Biol. Med. 110, 879–881 (1962).
Barton, E. S. et al. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Invest. 111, 1823–1833 (2003). This study demonstrates a linkage between sialic acid binding and alterations in reovirus tropism.
Frierson, J. M. et al. Utilization of sialylated glycans as coreceptors enhances the neurovirulence of serotype 3 reovirus. J. Virol. 86, 13164–13173 (2012). This work shows that sialic acid-binding capacity increases reovirus-induced neurovirulence in mice.
Konopka-Anstadt, J. L. et al. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe 15, 681–691 (2014).
Weiner, H. L., Drayna, D., Averill, D. R. Jr & Fields, B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc. Natl Acad. Sci. USA 74, 5744–5748 (1977).
Weiner, H. L., Greene, M. I. & Fields, B. N. Delayed hypersensitivity in mice infected with reovirus. I. Identification of host and viral gene products responsible for the immune response. J. Immunol. 125, 278–282 (1980).
Morrison, L. A., Sidman, R. L. & Fields, B. N. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc. Natl Acad. Sci. USA 88, 3852–3856 (1991).
Tyler, K. L., McPhee, D. A. & Fields, B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science 233, 770–774 (1986).
Cupelli, K. & Stehle, T. Viral attachment strategies: the many faces of adenoviruses. Curr. Opin. Virol. 1, 84–91 (2011).
Kemp, M. C., Hierholzer, J. C., Cabradilla, C. P. & Obijeski, J. F. The changing etiology of epidemic keratoconjunctivitis: antigenic and restriction enzyme analyses of adenovirus types 19 and 37 isolated over a 10-year period. J. Infect. Dis. 148, 24–33 (1983).
Arnberg, N., Edlund, K., Kidd, A. H. & Wadell, G. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74, 42–48 (2000).
Wadell, G. Hemagglutination with adenovirus serotypes belonging to Rosen's subgroups II and 3. Proc. Soc. Exp. Biol. Med. 132, 413–421 (1969).
Burmeister, W. P., Guilligay, D., Cusack, S., Wadell, G. & Arnberg, N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 78, 7727–7736 (2004).
Spjut, S. et al. A potent trivalent sialic acid inhibitor of adenovirus type 37 infection of human corneal cells. Angew. Chem. Int. Ed. Engl. 50, 6519–6521 (2011). This paper reports the development of a multivalent inhibitor of viral infection that was inspired by the crystal structure of the Ad37 fibre knob in complex with its carbohydrate receptor; the inhibitor is more potent than monovalent sialic acid.
Yeager, M., Dryden, K. A., Olson, N. H., Greenberg, H. B. & Baker, T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J. Cell Biol. 110, 2133–2144 (1990).
Fiore, L., Greenberg, H. B. & Mackow, E. R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology 181, 553–563 (1991).
Denisova, E. et al. Rotavirus capsid protein VP5* permeabilizes membranes. J. Virol. 73, 3147–3153 (1999).
Dormitzer, P. R., Sun, Z. Y., Wagner, G. & Harrison, S. C. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21, 885–897 (2002). This study identifies the sialic acid-binding site of rhesus rotavirus and defines residues that are required for this glycan interaction.
Kraschnefski, M. J. et al. Effects on sialic acid recognition of amino acid mutations in the carbohydrate-binding cleft of the rotavirus spike protein. Glycobiology 19, 194–200 (2009).
Blanchard, H., Yu, X., Coulson, B. S. & von Itzstein, M. Insight into host cell carbohydrate-recognition by human and porcine rotavirus from crystal structures of the virion spike associated carbohydrate-binding domain (VP8*). J. Mol. Biol. 367, 1215–1226 (2007).
Yu, X. et al. Structural basis of rotavirus strain preference toward N-acetyl- or N-glycolylneuraminic acid-containing receptors. J. Virol. 86, 13456–13466 (2012).
Monnier, N. et al. High-resolution molecular and antigen structure of the VP8* core of a sialic acid-independent human rotavirus strain. J. Virol. 80, 1513–1523 (2006).
Haselhorst, T. et al. Recognition of the GM3 ganglioside glycan by Rhesus rotavirus particles. Angew. Chem. Int. Ed. Engl. 50, 1055–1058 (2011).
Haselhorst, T. et al. Sialic acid dependence in rotavirus host cell invasion. Nature Chem. Biol. 5, 91–93 (2009).
Sowmyanarayanan, T. V. et al. Severity of rotavirus gastroenteritis in Indian children requiring hospitalization. Vaccine 30 (Suppl. 1), 167–172 (2012).
Huang, P. et al. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J. Virol. 86, 4833–4843 (2012).
Liu, Y. et al. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. J. Virol. 86, 9899–9910 (2012).
Martinez, M. A., Lopez, S., Arias, C. F. & Isa, P. Gangliosides have a functional role during rotavirus cell entry. J. Virol. 87, 1115–1122 (2013).
Yolken, R. H., Willoughby, R., Wee, S. B., Miskuff, R. & Vonderfecht, S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J. Clin. Invest. 79, 148–154 (1987).
Kaufmann, J. K. & Nettelbeck, D. M. Virus chimeras for gene therapy, vaccination, and oncolysis: adenoviruses and beyond. Trends Mol. Med. 18, 365–376 (2012).
Russell, S. J., Peng, K. W. & Bell, J. C. Oncolytic virotherapy. Nature Biotech. 30, 658–670 (2012).
von Itzstein, M. et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363, 418–423 (1993).
Kim, C. U. et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 119, 681–690 (1997).
Russell, R. J. et al. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443, 45–49 (2006).
Kim, J. H. et al. Mechanism-based covalent neuraminidase inhibitors with broad-spectrum influenza antiviral activity. Science 340, 71–75 (2013).
Takemoto, D. K., Skehel, J. J. & Wiley, D. C. A surface plasmon resonance assay for the binding of influenza virus hemagglutinin to its sialic acid receptor. Virology 217, 452–458 (1996).
Matrosovich, M. & Klenk, H. D. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 13, 85–97 (2003).
Hendricks, G. L. et al. Sialylneolacto-N-tetraose c (LSTc)-bearing liposomal decoys capture influenza A virus. J. Biol. Chem. 288, 8061–8073 (2013). This study shows that liposomes that are coated with LSTc bind to influenza virus and function as antiviral agents.
Malakhov, M. P. et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob. Agents Chemother. 50, 1470–1479 (2006).
Belser, J. A. et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J. Infect. Dis. 196, 1493–1499 (2007).
Wathen, M. W., Barro, M. & Bright, R. A. Antivirals in seasonal and pandemic influenza — future perspectives. Influenza Other Respir. Viruses 7 (Suppl. 1), 76–80 (2013).
Hashiro, G., Loh, P. C. & Yau, J. T. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch. Virol. 54, 307–315 (1977).
Norman, K. L., Hirasawa, K., Yang, A. D., Shields, M. A. & Lee, P. W. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc. Natl Acad. Sci. USA 101, 11099–11104 (2004).
Strong, J. E., Coffey, M. C., Tang, D., Sabinin, P. & Lee, P. W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17, 3351–3362 (1998).
Coffey, M. C., Strong, J. E., Forsyth, P. A. & Lee, P. W. Reovirus therapy of tumors with activated Ras pathway. Science 282, 1332–1334 (1998).
Gollamudi, R. et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest. New Drugs 28, 641–649 (2009).
Vidal, L. et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin. Cancer. Res. 14, 7127–7137 (2008).
Sahin, E., Egger, M., McMasters, K. & Zhou, H. Development of oncolytic reovirus for cancer therapy. J. Cancer Ther. 4, 1100–1115 (2013).
Kyula, J. N., Roulstone, V., Karapanagiotou, E. M., Melcher, A. A. & Harrington, K. J. Oncolytic reovirus type 3 (Dearing) as a novel therapy in head and neck cancer. Expert Opin. Biol. Ther. 12, 1669–1678 (2012).
Raval, G. et al. TNF-α induction of GM2 expression on renal cell carcinomas promotes T cell dysfunction. J. Immunol. 178, 6642–6652 (2007).
Harduin-Lepers, A. et al. Sialyltransferases functions in cancers. Frontiers Biosci. 4, 499–515 (2012).
Kim, M. et al. Attenuated reovirus displays oncolysis with reduced host toxicity. Br. J. Cancer 104, 290–299 (2011).
Shinya, K. et al. Avian flu: influenza virus receptors in the human airway. Nature 440, 435–436 (2006).
Baum, L. G. & Paulson, J. C. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40, 35–38 (1990).
Walther, T. et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 9, e1003223 (2013). The authors use glycomics approaches to improve understanding of the glycome on human lung tissue and compare these findings with results from traditional glycan array experiments.
Comelli, E. M. et al. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology 16, 117–131 (2006).
Song, X. et al. Shotgun glycomics: a microarray strategy for functional glycomics. Nature Meth. 8, 85–90 (2011).
Byrd-Leotis, L. et al. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc. Natl Acad. Sci. USA 111, E2241–E2250 (2014). This study demonstrates how shotgun glycomics can be used to identify influenza virus receptors.
Acknowledgements
The authors' work was supported by US Public Health Service awards R01 AI76983 and R37 AI38296 and the Elizabeth B. Lamb Center for Pediatric Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Glycans
-
A nonspecific term for a polysaccharide or polymeric carbohydrate.
- α-linked
-
A term used to describe a Neu5Ac that is incorporated into a polysaccharide via a glycosidic bond in which the alpha anomer, or C1 carbon, of Neu5Ac is in the axial position on the opposite side of the plane of the C6 carbon.
- Lectins
-
Proteins, usually of plant origin, that bind to carbohydrates on the surface of animal cells; they also agglutinate red blood cells.
- Neuraminidases
-
Enzymes, usually of microbial origin, that catalyse the removal of terminal sialic acids on the surface of cells or microorganisms.
- Gangliosides
-
A type of glycolipid, commonly composed of a ceramide tail and a glycan portion that contains at least one sialic acid moiety.
- Serotypes
-
A subclassification of a virus species that shares antigens and for which antibodies are cross-reactive.
Rights and permissions
About this article
Cite this article
Stencel-Baerenwald, J., Reiss, K., Reiter, D. et al. The sweet spot: defining virus–sialic acid interactions. Nat Rev Microbiol 12, 739–749 (2014). https://doi.org/10.1038/nrmicro3346
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro3346
This article is cited by
-
Potential biological functions and future perspectives of sialylated milk oligosaccharides
Archives of Pharmacal Research (2024)
-
Material-engineered bioartificial microorganisms enabling efficient scavenging of waterborne viruses
Nature Communications (2023)
-
Sialic acid O-acetylation patterns and glycosidic linkage type determination by ion mobility-mass spectrometry
Nature Communications (2023)
-
Paired immunoglobulin-like receptor B is an entry receptor for mammalian orthoreovirus
Nature Communications (2023)
-
Fluorescent GD2 analog for single-molecule imaging
Glycoconjugate Journal (2023)