Abstract
The ultimate solution to the global HIV-1 epidemic will probably require the development of a safe and effective vaccine. Multiple vaccine platforms have been evaluated in preclinical and clinical trials, but given the disappointing results of clinical efficacy studies so far, novel vaccine approaches are needed. In this Opinion article, we discuss the scientific basis and clinical potential of novel adenovirus and cytomegalovirus vaccine vectors for HIV-1 as two contrasting but potentially complementary vector approaches. Both of these vector platforms have demonstrated partial protection against stringent simian immunodeficiency virus challenges in rhesus monkeys using different immunological mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
12 October 2017
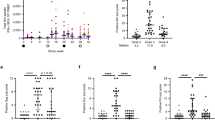
On page 768 of the article, the text has been changed to clarify that all vaccinated and control animals were challenged until documentation of SIV infection by virological and/or immunological criteria. Furthermore, on page 768, the text has been changed to specify both the number of vaccinated controllers shown in Figure 2 after intrarectal challenge as well as the number of controllers after both intrarectal and intravaginal challenge. Finally, the legend of Figure 2 has been changed to clarify that the graph shows intrarectal SIVmac239 challenge of monkeys that have either been vaccinated twice with a strain 68-1 RhCMV/SIV vector (week 0 and week 14; n = 36) or once with these vectors (week 0) and were then vaccinated at week 14 with Ad5 vectors (n = 12). Changes have been made to both the HTML and PDF versions of the article. The authors apologize to readers for any confusion caused.
References
Barouch, D. H. The quest for an HIV-1 vaccine — moving forward. N. Engl. J. Med. 369, 2073–2076 (2013).
Fauci, A. S. & Marston, H. D. Ending AIDS — is an HIV vaccine necessary? N. Engl. J. Med. 370, 495–498 (2014).
Buchbinder, S. P. et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893 (2008).
Flynn, N. M. et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191, 654–665 (2005).
Gray, G. E. et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 11, 507–515 (2011).
Hammer, S. M. et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 369, 2083–2092 (2013).
Pitisuttithum, P. et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194, 1661–1671 (2006).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 (2009).
Shiver, J. W. & Emini, E. A. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55, 355–372 (2004).
Shiver, J. W. et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415, 331–335 (2002).
Casimiro, D. R. et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79, 15547–15555 (2005).
Wilson, N. A. et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80, 5875–5885 (2006).
Benlahrech, A. et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl Acad. Sci. USA 106, 19940–19945 (2009).
Hutnick, N. A. et al. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nature Med. 15, 876–878 (2009).
McElrath, M. J. et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372, 1894–1905 (2008).
O'Brien, K. L. et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nature Med. 15, 873–875 (2009).
Letvin, N. L. et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3, ra36 (2011).
Lopker, M. et al. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J. Virol. 87, 5477–5492 (2013).
Barouch, D. H. et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29, 5203–5209 (2011).
Mast, T. C. et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28, 950–957 (2010).
Abbink, P. et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81, 4654–4663 (2007).
Baden, L. R. et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J. Infect. Dis. 207, 240–247 (2013).
Barouch, D. H. et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J. Infect. Dis. 207, 248–256 (2013).
Creech, C. B. et al. Randomized, placebo-controlled trial to assess the safety and immunogenicity of an adenovirus type 35-based circumsporozoite malaria vaccine in healthy adults. Hum. vaccin. Immunother. 9, 2548–2557 (2013).
Keefer, M. C. et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS ONE 7, e41936 (2012).
Vogels, R. et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77, 8263–8271 (2003).
Antrobus, R. D. et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. 22, 668–674 (2013).
Barnes, E. et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4, ra111 (2012).
Colloca, S. et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4, ra112 (2012).
O'Hara, G. A. et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J. Infect. Dis. 205, 772–781 (2012).
Li, H. et al. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J. Virol. 86, 10862–10865 (2012).
Waddington, S. N. et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409 (2008).
Perreau, M. et al. The number of Toll-like receptor 9-agonist motifs in the adenovirus genome correlates with induction of dendritic cell maturation by adenovirus immune complexes. J. Virol. 86, 6279–6285 (2012).
Quinn, K. M. et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 190, 2720–2735 (2013).
Teigler, J. E., Iampietro, M. J. & Barouch, D. H. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86, 9590–9598 (2012).
Penaloza-MacMaster, P. et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J. Virol. 87, 1373–1384 (2013).
Tan, W. G. et al. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 87, 1359–1372 (2013).
Liu, J. et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime–boost regimens in rhesus monkeys. J. Virol. 82, 4844–4852 (2008).
Barouch, D. H. et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–93 (2012).
Barouch, D. H. et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nature Med. 16, 319–323 (2010).
Fischer, W. et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nature Med. 13, 100–106 (2007).
Santra, S. et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nature Med. 16, 324–328 (2010).
Barouch, D. H. et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155, 531–539 (2013).
Baden, L. R. et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J. Infect. Dis. http://dx.doi.org/10.1093/infdis/jiu485 (2014).
Hansen, S. G. et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527 (2011).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013).
Hansen, S. G. et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature Med. 15, 293–299 (2009).
Hansen, S. G. et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328, 102–106 (2010).
Hansen, S. G. et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340, 1237874 (2013).
Bannard, O., Kraman, M. & Fearon, D. Pathways of memory CD8+ T-cell development. Eur. J. Immunol. 39, 2083–2087 (2009).
Genesca, M., McChesney, M. B. & Miller, C. J. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J. Intern. Med. 265, 67–77 (2009).
Picker, L. J. et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 116, 1514–1524 (2006).
Pitcher, C. J. et al. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168, 29–43 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Grossman, Z., Meier-Schellersheim, M., Paul, W. E. & Picker, L. J. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nature Med. 12, 289–295 (2006).
Champagne, P. et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410, 106–111 (2001).
Gauduin, M. C. et al. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J. Exp. Med. 203, 2661–2672 (2006).
Kern, F. et al. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29, 2908–2915 (1999).
Picker, L. J., Hansen, S. G. & Lifson, J. D. New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med. 63, 95–111 (2012).
Fruh, K., Malouli, D., Oxford, K. L. & Barry, P. A. in Cytomegalovirus Vol. 2. (ed. Reddehase,M. J.) (Caister Academic Press, 2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L.J.P and Oregon Health and Science University (OHSU) have a significant financial interest in TomegaVax, Inc., which is a company that may have a commercial interest in the results of this research and technology. The potential individual and institutional conflicts of interest have been reviewed and managed by OHSU. D.H.B. is a named co-inventor on various vector and antigen patents.
Glossary
- Anamnestic response
-
The enhanced immune response that occurs against an antigen as a result of previous host exposure to a related antigen.
- CD46
-
A ubiquitously expressed type-1 transmembrane protein that functions to regulate complement. It functions as a receptor for vaccine strains of measles virus and some adenovirus serotypes.
- Central memory T cell
-
(TCM cell). An antigen-experienced resting T cell that expresses cell surface receptors that are required for homing to secondary lymphoid organs. These cells are generally long-lived and can function as the precursors for effector T cells during recall responses.
- Coxsackie and adenovirus receptor
-
(CAR). An immunoglobulin-like transmembrane cell adhesion protein that is used by some coxsackievirus and adenovirus species as a receptor.
- Effector memory T cell
-
(TEM cell). An antigen-experienced T cell that maintains effector differentiation, resides in, or expresses cell surface receptors that are required for homing to, extra-lymphoid effector sites and that has limited expansion potential.
- Elite controller
-
A patient infected with HIV whose immune system can limit viral RNA to below 50 copies per ml for at least 12 months in the absence of highly active antiretroviral therapy.
- Heterologous prime–boost
-
Repeated immunization using different vaccines; it is used to stimulate a better immune response.
- Neutralizing antibodies
-
Antibodies that block infectivity (for example, of a virus), usually by binding to the foreign particle (the virion) and incapacitating it in some way.
Rights and permissions
About this article
Cite this article
Barouch, D., Picker, L. Novel vaccine vectors for HIV-1. Nat Rev Microbiol 12, 765–771 (2014). https://doi.org/10.1038/nrmicro3360
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro3360
This article is cited by
-
Lentiviral vector induces high-quality memory T cells via dendritic cells transduction
Communications Biology (2021)
-
RhCMV serostatus and vaccine adjuvant impact immunogenicity of RhCMV/SIV vaccines
Scientific Reports (2020)
-
Production of HIV-1-based virus-like particles for vaccination: achievements and limits
Applied Microbiology and Biotechnology (2019)
-
Bone marrow stem cells to destroy circulating HIV: a hypothetical therapeutic strategy
Journal of Biological Research-Thessaloniki (2018)
-
First Impressions—the Potential of Altering Initial Host-Virus Interactions for Rational Design of Herpesvirus Vaccine Vectors
Current Clinical Microbiology Reports (2018)