Key Points
-
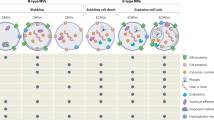
Extracellular vesicle (EV) research in Gram-positive bacteria, mycobacteria and fungi was neglected until recently, owing to the presumption that vesicles could not traverse the thick cell walls found in these organisms.
-
EVs are now understood to be produced by all types of microorganism, including those with thick cell walls, and are biologically active.
-
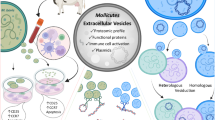
EVs from bacteria, mycobacteria and fungi contain virulence factors, such as toxins, that are involved in pathogenesis and elicit strong host immune responses. For example, Cryptococcus neoformans EVs carry the capsular polysaccharide glucuronoxylomannan, which is an important virulence factor.
-
Interaction of EVs with the host is specific to the microorganism from which the EVs were produced and is based on the lipid content and cargo of the EVs.
-
Research into EVs produced by microorganisms with thick cell walls is a very young field. By learning how these microorganisms use EVs, we hope that researchers will gain insight into pathogenesis, therapeutics and vaccines.
Abstract
Extracellular vesicles (EVs) are produced by all domains of life. In Gram-negative bacteria, EVs are produced by the pinching off of the outer membrane; however, how EVs escape the thick cell walls of Gram-positive bacteria, mycobacteria and fungi is still unknown. Nonetheless, EVs have been described in a variety of cell-walled organisms, including Staphylococcus aureus, Mycobacterium tuberculosis and Cryptococcus neoformans. These EVs contain varied cargo, including nucleic acids, toxins, lipoproteins and enzymes, and have important roles in microbial physiology and pathogenesis. In this Review, we describe the current status of vesiculogenesis research in thick-walled microorganisms and discuss the cargo and functions associated with EVs in these species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rodrigues, M. L. et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7, 58–67 (2008).
Albuquerque, P. C. et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 10, 1695–1710 (2008).
Lee, E. et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436 (2009). This article is the first extensive proteomic study of EVs produced by Gram-positive bacteria.
Rivera, J. et al. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl Acad. Sci. 107, 19002–19007 (2010).
Gurung, M. et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 6, e27958 (2011).
Prados-Rosales, R. et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121, 1471–1483 (2011). This report is the first to describe the production of EVs in the two most medically important species of mycobacteria, M. tuberculosis and M. bovis BCG, stating that EVs from pathogenic strains of mycobacteria concentrate lipoproteins and modulate the immune response in a TLR2-dependent manner.
Lee, J. et al. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob. Agents Chemother. 57, 2589–2595 (2013).
Lee, J. H. et al. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE 8, e73196 (2013).
Olaya-Abril, A. et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteomics 106, 46–60 (2014).
Brown, L., Kessler, A., Cabezas-Sanchez, P., Luque-Garcia, J. L. & Casadevall, A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol. Microbiol. 93, 183–198 (2014).
Jiang, Y., Kong, Q., Roland, K. L. & Curtiss, R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int. J. Med. Microbiol. 304, 431–443 (2014).
Rodrigues, M. L., Nakayasu, E. S., Almeida, I. C. & Nimrichter, L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J. Proteomics 97, 177–186 (2014).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012). This review extensively covers EV biogenesis and function in bacteria, eukaryotes and archaea.
Bishop, D. G. & Work, E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 96, 567–576 (1965).
Knox, K. W., Vesk, M. & Work, E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J. Bacteriol. 92, 1206–1217 (1966).
Work, E., Knox, K. W. & Vesk, M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann. N. Y. Acad. Sci. 133, 438–449 (1966). This paper reports some of the first TEM images of what have since been identified as OMVs from Gram-negative bacteria.
Knox, K. W., Cullen, J. & Work, E. An extracellular lipopolysaccharide–phospholipid–protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 103, 192–201 (1967).
Takeo, K., Uesaka, I., Uehira, K. & Nishiura, M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J. Bacteriol. 113, 1442–1448 (1973).
Dorward, D. W. & Garon, C. F. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl. Environ. Microbiol. 56, 1960–1962 (1990).
Scwechheimer, C. & Kuehn, M. Outer membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. http://dx.doi.org/10.1038/nrmicro3525, (2015).
Kuehn, M. J. & Kesty, N. C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19, 2645–2655 (2005).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Gyorgy, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
Andaloussi, S. E. L., Mager, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
Acevedo, R. et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 5, 121 (2014).
Beveridge, T. J. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181, 4725–4733 (1999).
Ellis, T. N. & Kuehn, M. J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 (2010).
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 (2010).
Costerton, J. W., Ingram, J. M. & Cheng, K. J. Structure and function of the cell envelope of Gram-negative bacteria. Bacteriol. Rev. 38, 87–110 (1974).
Schertzer, J. W. & Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 3, e00297-11 (2012).
Kulkarni, H. M. & Jagannadham, M. V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 160, 2109–2121 (2014).
Shockman, G. D. & Barrett, J. F. Structure, function, and assembly of cell walls of Gram-positive bacteria. Annu. Rev. Microbiol. 37, 501–527 (1983).
Brennan, P. J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83, 91–97 (2003).
Bowman, S. M. & Free, S. J. The structure and synthesis of the fungal cell wall. Bioessays 28, 799–808 (2006).
Free, S. J. Fungal cell wall organization and biosynthesis. Adv. Genet. 81, 33–82 (2013).
Rodrigues, M. L. et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6, 48–59 (2007). This study definitively identifies and characterizes EVs from fungi.
Marsollier, L. et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3, e62 (2007). This work associates the toxin mycolactone with EVs and is the first research into EVs in mycobacteria.
Prados-Rosales, R., Brown, L., Casadevall, A., Montalvo-Quirós, S. & Luque-Garcia, J. L. Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria. MethodsX 1, 124–129 (2014).
Tjalsma, H. et al. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233 (2004).
Ligon, L. S., Hayden, J. D. & Braunstein, M. The ins and outs of Mycobacterium tuberculosis protein export. Tuberculosis 92, 121–132 (2012).
Delic, M. et al. The secretory pathway: exploring yeast diversity. FEMS Microbiol. Rev. 37, 872–914 (2013).
Tjalsma, H., Bolhuis, A., Jongbloed, J. D., Bron, S. & van Dijl, J. M. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64, 515–547 (2000).
Gomez, M., Johnson, S. & Gennaro, M. L. Identification of secreted proteins of Mycobacterium tuberculosis by a bioinformatic approach. Infect. Immun. 68, 2323–2327 (2000).
Vallejo, M. C. et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J. Proteome Res. 11, 1676–1685 (2012).
Sorgo, A. G., Heilmann, C. J., Brul, S., de Koster, C. G. & Klis, F. M. Beyond the wall: Candida albicans secret(e)s to survive. FEMS Microbiol. Lett. 338, 10–17 (2013).
Anderson, J., Mihalik, R. & Soll, D. R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J. Bacteriol. 172, 224–235 (1990).
Vargas, G. et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell. Microbiol. 17, 389–407 (2015).
Schrempf, H., Koebsch, I., Walter, S., Engelhardt, H. & Meschke, H. Extracellular Streptomyces vesicles: amphorae for survival and defence. Microb. Biotechnol. 4, 286–299 (2011).
Hong, S. W. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66, 351–359 (2011). This investigation shows that S. aureus EVs are biologically active and cause atopic dermatitis.
Rath, P. et al. Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 110, E4790–E4797 (2013). This article describes the first gene shown to regulate vesiculogenesis in M. tuberculosis (virR ); a mutant deficient in VirR overproduces EVs and is attenuated in virulence, suggesting that vesiculogenesis is important for Mycobacterium spp. pathogenesis.
Thay, B., Wai, S. N. & Oscarsson, J. Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 8, e54661 (2013).
Prados-Rosales, R. et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol. 196, 1250–1256 (2014).
Liao, S. et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 196, 2355–2366 (2014).
Prados-Rosales, R. et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. mBio 5, e01921-14 (2014).
Wolf, J. M., Rivera, J. & Casadevall, A. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell. Microbiol. 14, 762–773 (2012). This study shows that EVs from Gram-positive bacteria and fungi can be disrupted by albumin.
Hristov, M., Erl, W., Linder, S. & Weber, P. C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104, 2761–2766 (2004).
Oliveira, D. L. et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE 5, e11113 (2010).
Vallejo, M. C. et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-galactosyl epitopes. Eukaryot. Cell 10, 343–351 (2011).
Vallejo, M. C. et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS ONE 7, e39463 (2012).
Klis, F. M., de Koster, C. G. & Brul, S. Cell wall-related bionumbers and bioestimates of Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell 13, 2–9 (2014).
Doering, T. L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 63, 223–247 (2009).
Gow, N. A. & Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 15, 406–412 (2012).
Levin, D. E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189, 1145–1175 (2011).
de Souza Pereira, R. & Geibel, J. Direct observation of oxidative stress on the cell wall of Saccharomyces cerevisiae strains with atomic force microscopy. Mol. Cell. Biochem. 201, 17–24 (1999).
Jacobson, E. S. & Ikeda, R. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. 43, 327–333 (2005).
Wolf, J., Espadas-Moreno, J., Luque-Garcia, J. L. & Casadevall, A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 13, 1484–1493 (2014).
Kopecka, M., Yamaguchi, M., Gabriel, M., Takeo, K. & Svoboda, A. Morphological transitions during the cell division cycle of Cryptococcus neoformans as revealed by transmission electron microscopy of ultrathin sections and freeze-substitution. Scripta Med. 73, 369–380 (2000).
McBroom, A. J., Johnson, A. P., Vemulapalli, S. & Kuehn, M. J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188, 5385–5392 (2006).
Ollinger, J., Bowen, B., Wiedmann, M., Boor, K. J. & Bergholz, T. M. Listeria monocytogenes σB modulates PrfA-mediated virulence factor expression. Infect. Immun. 77, 2113–2124 (2009).
Parida, S. K. et al. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol. 28, 81–93 (1998).
Nicola, A. M., Frases, S. & Casadevall, A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot. Cell 8, 1373–1380 (2009).
da Silva, R. P. et al. Extracellular vesicle-mediated export of fungal RNA. Sci. Rep. 5, 7763 (2015).
Dubois, J. Y. et al. Immunity to the bacteriocin sublancin 168 Is determined by the SunI (YolF) protein of Bacillus subtilis. Antimicrob. Agents Chemother. 53, 651–661 (2009).
Rossi, J., Bischoff, M., Wada, A. & Berger-Bachi, B. MsrR, a putative cell envelope-associated element involved in Staphylococcus aureus sarA attenuation. Antimicrob. Agents Chemother. 47, 2558–2564 (2003).
Bantel, H. et al. α-toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155, 637–648 (2001).
Casadevall, A., Rosas, A. L. & Nosanchuk, J. D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3, 354–358 (2000).
Zhu, X. & Williamson, P. R. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 5, 1–10 (2004).
Eisenman, H. C. et al. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153, 3954–3962 (2007).
Khajo, A. et al. Protection of melanized Cryptococcus neoformans from lethal dose γ irradiation involves changes in melanin's chemical structure and paramagnetism. PLoS ONE 6, e25092 (2011).
Portnoy, D. A., Jacks, P. S. & Hinrichs, D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167, 1459–1471 (1988).
Cossart, P. et al. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57, 3629–3636 (1989).
Hirst, R. A. et al. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 197, 744–751 (2008).
Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35 (2003).
Smith, H., Keppie, J. & Stanley, J. L. The chemical basis of the virulence of Bacillus anthracis. V. The specific toxin produced by B. anthracis in vivo. Br. J. Exp. Pathol. 36, 460–472 (1955).
Mosser, E. M. & Rest, R. F. The Bacillus anthracis cholesterol-dependent cytolysin, Anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 6, 56 (2006).
Macfarlane, M. G. & Knight, B. C. The biochemistry of bacterial toxins: the lecithinase activity of Cl. welchii toxins. Biochem. J. 35, 884–902 (1941).
Keyburn, A., Bannam, T., Moore, R. & Rood, J. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2, 1913–1927 (2010).
Snyder, D. S. & Small, P. L. Uptake and cellular actions of mycolactone, a virulence determinant for Mycobacterium ulcerans. Microb. Pathog. 34, 91–101 (2003).
Oliveira, D. L. et al. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 78, 1601–1609 (2010).
Monari, C. et al. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J. Immunol. 174, 3461–3468 (2005).
Monari, C. et al. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 191, 127–137 (2005).
Drage, M. G. et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 258, 29–37 (2009).
Huang, S. H. et al. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS ONE 7, e48570 (2012).
Bansal, K. et al. PIM2 Induced COX-2 and MMP-9 expression in macrophages requires PI3K and Notch1 signaling. PLoS ONE 4, e4911 (2009).
Bryers, J. D. Medical biofilms. Biotechnol. Bioeng. 100, 1–18 (2008).
Robertson, E. J., Wolf, J. M. & Casadevall, A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl. Environ. Microbiol. 78, 7977–7984 (2012).
Eisenman, H. C., Frases, S., Nicola, A. M., Rodrigues, M. L. & Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155, 3860–3867 (2009).
Walker, C. A. et al. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot. Cell 9, 1329–1342 (2010).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13, 1554–1571 (2013).
Kim, D. K. et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2, 20384 (2013).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 34, 474–490 (2014).
Kim, D. K. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31, 933–939 (2015).
Fernandez, S. et al. A proteoliposome formulation derived from Bordetella pertussis induces protection in two murine challenge models. BMC Immunol. 14, S8 (2013).
Holst, J. et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27 (Suppl. 2), B3–B12 (2009).
Kadurugamuwa, J. L. & Beveridge, T. J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177, 3998–4008 (1995).
Kadurugamuwa, J. L. & Beveridge, T. J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178, 2767–2774 (1996).
Mashburn-Warren, L. M. & Whiteley, M. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61, 839–846 (2006).
Kolling, G. L. & Matthews, K. R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 65, 1843–1848 (1999).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Bomberger, J. M. et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 (2009).
Bernheimer, A. W. & Avigad, L. S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J. Gen. Microbiol. 61, 361–369 (1970).
Heerklotz, H. & Seelig, J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys. J. 81, 1547–1554 (2001).
Carrillo, C., Teruel, J. A., Aranda, F. J. & Ortiz, A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim. Biophys. Acta 1611, 91–97 (2003).
Nakano, M. M., Marahiel, M. A. & Zuber, P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170, 5662–5668 (1988).
Quadri, L. E. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 170, 1585–1595 (1998).
Acknowledgements
A.C. was supported by the US National Institutes of Health (awards 5R01HL059842, 5R01AI033774, 5R37AI033142 and 5R01AI052733). R.P.-R. was supported in part by the US National Institutes of Health (award 1R21AI115091).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Virulence factors
-
Products that are important for infection by and survival of a pathogen. These factors can include adhesins, DNA, toxins and other molecules.
- Biofilms
-
Surface-dwelling bacterial cultures that are highly resistant to disruption and removal. Biofilms can consist of a single species or a community of multiple microorganisms.
- Cell wall
-
The rigid structure that surrounds a microorganism and has a role in cell shape and homeostasis.
- Melanin
-
A pigmented molecule derived from laccase-assisted oxidation of dihydroxy phenol compounds. Melanin can protect cells against oxidative damage.
- Multivesicular bodies
-
(MVBs). A subset of endosomes that contain membrane-bound intraluminal vesicles that originate by budding into the MVB. Typically, MVBs fuse with the cell membrane to release the intraluminal vesicles into the extracellular space.
- B-band lipopolysaccharide
-
(B-band LPS). LPS that is highly charged at neutral pH owing to the presence of a large number of phosphate groups and long O side chains, in contrast to Aband LPS.
- Siderophores
-
Iron-scavenging molecules produced by microorganisms. Some microorganisms can also steal siderophores from other microorganisms in order to obtain iron.
- Basidiomycetes
-
Filamentous fungi that reproduce sexually via the formation of specialized cells called basidia, which bear external spores called basidiospores. Some basidiomycetes can also reproduce asexually.
- Ascomycetes
-
Fungi that, when reproducing sexually, form a structure called an ascus in which the spores are formed. Some ascomycetes can also reproduce asexually.
- Extracellular DNA
-
(eDNA). DNA that is present in the extracellular milieu and might function in intercellular communication. eDNA can also be a structural component of biofilms and neutrophil extracellular traps.
- Penicillin-binding proteins
-
Proteins that are essential for bacterial cell wall biogenesis and also have the capacity to bind to penicillin.
- Glucuronoxylomannan
-
(GXM). A polysaccharide that is produced by the pathogenic fungus Cryptococcus neoformans and is a major component of the cellular capsule.
- Cytolysin
-
A toxin with the ability to lyse cells.
- Mycolactone
-
A macrolide toxin that is produced by a group of mycobacteria and causes Buruli ulcers in humans. It is required for virulence, is cytotoxic and blocks the translocation of immune proteins into the endoplasmic reticulum as a mechanism of immunosuppression.
- Cholesterol microdomains
-
Lipid domains in the cellular lipid bilayer that are enriched in cholesterol.
- T helper 2 cell
-
(TH2 cells). A subset of CD4+ T cells that is of paramount importance for host defence against extracellular pathogens. TH2 cells secrete the cytokines interleukin-4 (IL-4), IL-5, IL-6, IL-9, IL-10 and IL-13, leading to strong antibody responses.
- TH1 cell
-
A subset of CD4+ effector T cells that is required for host defence against intracellular viral and bacterial pathogens. TH1 cells secrete cytokines such as interferon-γ (IFNγ), interleukin-2 (IL-2), IL-10 and lymphotoxin, promoting macrophage activation, nitric oxide production and cytotoxic T lymphocyte proliferation.
- Granulomatous inflammation
-
The aggregation of mononuclear inflammatory cells, which can be accompanied by the infiltration of other leukocytes or by necrosis.
- Koch phenomenon
-
A rapid inflammatory response that develops to a reinfection with Mycobacterium tuberculosis and that is marked by necrotic lesions. The response is caused by hypersensitivity to products of the tubercle bacillus.
- Proteoliposomes
-
Synthetic liposomes with proteins embedded into the lipid bilayer.
- Lipidic nanovesicles
-
Nanoscale lipid-bilayer spheres or liposomes.
Rights and permissions
About this article
Cite this article
Brown, L., Wolf, J., Prados-Rosales, R. et al. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13, 620–630 (2015). https://doi.org/10.1038/nrmicro3480
Published:
Issue date:
DOI: https://doi.org/10.1038/nrmicro3480
This article is cited by
-
Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields
Cell Communication and Signaling (2024)
-
Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes
Infectious Agents and Cancer (2023)
-
Bacillus cereus extracellular vesicles act as shuttles for biologically active multicomponent enterotoxins
Cell Communication and Signaling (2023)
-
Emerging role of bacterial outer membrane vesicle in gastrointestinal tract
Gut Pathogens (2023)
-
F. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice
Microbial Cell Factories (2023)