Key Points
-
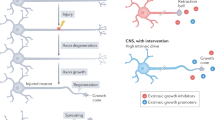
The fact that the adult mammalian central nervous system (CNS) does not regenerate after injury was recognized as early as 1550 BC. There are two main obstacles to regeneration — inhibitors within myelin, and the formation of a glial scar. This review will discuss what is known about the myelin inhibitors, and how their effects might be overcome.
-
Three inhibitors of axonal regeneration have been identified in myelin – Nogo, myelin-associated glycoprotein (Mag) and oligodendrocyte myelin glycoprotein (Omgp). All of these proteins induce growth cone collapse and inhibit neurite outgrowth.
-
In 2001, Strittmatter and colleagues cloned the Nogo receptor (Ngr), which they identified as a binding partner of the Nogo domain Nogo-66. It has since been found that Mag and Omgp also bind to this receptor. Ngr cannot transduce signals across the membrane, as it has no transmembrane and cytoplasmic domains, so it seems to use the p75 neurotrophin receptor (p75NTR) as a transducing partner.
-
Members of the Rho family of small GTPases have been implicated in myelin's inhibitory effects. Rho has been suggested to interact directly with p75NTR, and activation of p75NTR initiates various signalling cascades. Some of these cascades might run in parallel with the Rho pathway to bring about inhibition when myelin inhibitors bind to the Ngr–p75NTR complex.
-
Two approaches might be used to overcome inhibitors and encourage regeneration. First, the inhibitors and/or their receptors could be blocked with antibodies or peptides. Second, the intrinsic state of the neuron could be changed, such that it no longer recognizes the environment as inhibitory. Elevation of cyclic AMP inside the injured neuron has been shown to overcome inhibition by Mag and myelin.
-
To identify genes and proteins that are required for regeneration, it is useful to look for genes that are upregulated in systems that regenerate spontaneously after injury. Studies have focused on two such situations — neonatal spinal neurons, which can regenerate to produce complete functional recovery, and regeneration of the CNS branch of dorsal root ganglion neurons after the peripheral branch has been lesioned.
-
Overcoming inhibitors of regeneration in myelin is only one aspect of a complex problem. Even if axons can be induced to grow across the lesion site before the glial scar forms, they still need to be directed back to their correct destination and make functional synapses. The first step is to get the axons to grow, and the last few years have seen enormous advances in this respect.
Abstract
Recent studies have expanded our knowledge, at the molecular level, of how myelin inhibits axonal regeneration after injury to the mammalian central nervous system. Several inhibitors have been identified that seem to signal inhibition through the same receptor complex. New molecular information has also accumulated on how the neuron can be changed intrinsically to overcome myelin inhibitors. Together, these important advances in the field have identified many new targets for therapeutic intervention to encourage nerve regeneration after spinal cord or brain injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fitch, M. T. & Silver, J. in CNS Regeneration (eds Tuszynski, M. H. & Kordower, J. H.) 55–88 (Academic, San Diego, 1999).
Qiu, J., Cai, D. & Filbin, M. T. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia 29, 166–174 (2000).
Schwab, M. E. & Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76, 319–370 (1996).
Huang, D. W., McKerracher, L., Braun, P. E. & David, S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron 24, 639–647 (1999).
Ramón y Cajal, S. Cajal's Degeneration & Regeneration of the Nervous System (eds DeFelipe, J. & Jones, E. G.) (Oxford Univ. Press, Oxford, 1928).
Berry, M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl. Anat. 23, 1–11 (1982). The first experimental data that support the suggestion that myelin inhibits axonal regeneration.
Crutcher, K. A. Tissue sections from the mature rat brain and spinal cord as substrates for neurite outgrowth in vitro: extensive growth on gray matter but little growth on white matter. Exp. Neurol. 104, 39–54 (1989).
Caroni, P. & Schwab, M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 106, 1281–1288 (1988).
Caroni, P. & Schwab, M. E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1, 85–96 (1988). References 8 and 9 identify inhibitory fractions in myelin and describe the IN-1 monoclonal antibody that neutralizes the inhibition.
Schnell, L. & Schwab, M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, 269–272 (1990).
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S. M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 (2000).
Prinjha, R. et al. Inhibitor of neurite outgrowth in humans. Nature 403, 383–384 (2000). References 11–13 describe the cloning of Nogo, a long-sought antigen of the IN-1 monoclonal antibody.
Huber, A. B., Weinmann, O., Brosamle, C., Oertle, T. & Schwab, M. E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 22, 3553–3567 (2002).
Wang, X. et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J. Neurosci. 22, 5505–15 (2002).
McKerracher, L. et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13, 805–811 (1994).
Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, P. R. & Filbin, M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13, 757–767 (1994).
Cai, D. et al. Neuronal cyclic amp controls the developmental loss in ability of axons to regenerate. J. Neurosci. 21, 4731–4739 (2001).
de Bellard, M. & Filbin, M. T. Myelin-associated glycoprotein, MAG, selectively binds several neuronal proteins. J. Neurosci. Res. 56, 213–218 (1999).
Turnley, A. M. & Bartlett, P. F. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport 9, 1987–1990 (1998).
Johnson, P. W. et al. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron 3, 377–385 (1989).
Salzer, J. L., Holmes, W. P. & Colman, D. R. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J. Cell Biol. 104, 957–965 (1987).
Salzer, J. L. et al. Structure and function of the myelin-associated glycoproteins. Ann. NY Acad. Sci. 605, 302–312 (1990).
Kelm, S. et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 (1994).
Tang, S. et al. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J. Cell Biol. 138, 1355–1366 (1997).
Trapp, B. D. Distribution of the myelin-associated glycoprotein and P0 protein during myelin compaction in quaking mouse peripheral nerve. J. Cell Biol. 107, 675–685 (1988).
Willison, H. J. et al. Myelin-associated glycoprotein and related glycoconjugates in developing cat peripheral nerve: a correlative biochemical and morphometric study. J. Neurochem. 49, 1853–1862 (1987).
Wang, K. C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
Habib, A. A. et al. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J. Neurochem. 70, 1704–1711 (1998).
Mikol, D. D., Gulcher, J. R. & Stefansson, K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J. Cell Biol. 110, 471–479 (1990).
Kottis, V. et al. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 82, 1566–1569 (2002).
Fournier, A. E., GrandPre, T. & Strittmatter, S. M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001). Identifies the first receptor for an inhibitor found in myelin.
Josephson, A. et al. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J. Comp. Neurol. 453, 292–304 (2002).
Oertle, T. et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 23, 5393–5406 (2003).
Liu, B. P., Fournier, A., GrandPre, T. & Strittmatter, S. M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297, 1190–1193 (2002).
Domeniconi, M. et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–290 (2002). References 35 and 36 reveal, surprisingly, that Mag interacts with the Ngr, and reference 34 shows that OMgp also interacts with this receptor.
Yamashita, T., Higuchi, H. & Tohyama, M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 157, 565–570 (2002).
Wang, K. C., Kim, J. A., Sivasankaran, R., Segal, R. & He, Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420, 74–78 (2002).
Wong, S. T. et al. A p75NTR and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nature Neurosci. 5, 1302–1308 (2002). References 37–39 identify the p75NTR as a transducing partner of Ngr.
He, X. L. et al. Structure of the Nogo receptor ectodomain. A recognition module implicated in myelin inhibition. Neuron 38, 177–185 (2003).
Bartsch, U. et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15, 1375–1381 (1995).
Li, M. et al. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J. Neurosci. Res. 46, 404–414 (1996).
Bregman, B. S. et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378, 498–501 (1995).
Schnell, L. & Schwab, M. E. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur. J. Neurosci. 5, 1156–1171 (1993).
GrandPre, T., Li, S. & Strittmatter, S. M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417, 547–551 (2002).
Kim, J. E., Li, S., GrandPre, T., Qiu, D. & Strittmatter, S. M. Axon regeneration in young adult mice lacking nogo-a/b. Neuron 38, 187–199 (2003).
Simonen, M. et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211 (2003).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003). References 46–48 describe different phenotypes when Nogo is knocked out.
Trapp, B. D. Myelin-associated glycoprotein. Location and potential functions. Ann. NY Acad. Sci. 605, 29–43 (1990).
Apostolski, S. et al. Identification of Gal(β1–3)GalNAc bearing glycoproteins at the nodes of Ranvier in peripheral nerve. J. Neurosci. Res. 38, 134–141 (1994).
Josephson, A., Widenfalk, J., Widmer, H. W., Olson, L. & Spenger, C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp. Neurol. 169, 319–328 (2001).
McMahon, S. B., Armanini, M. P., Ling, L. H. & Phillips, H. S. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12, 1161–1171 (1994).
Wright, D. E. & Snider, W. D. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J. Comp. Neurol. 351, 329–338 (1995).
Ernfors, P., Henschen, A., Olson, L. & Persson, H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron 2, 1605–1613 (1989).
Koliatsos, V. E., Crawford, T. O. & Price, D. L. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 549, 297–304 (1991).
Walsh, G. S., Krol, K. M., Crutcher, K. A. & Kawaja, M. D. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin receptor. J. Neurosci. 19, 4155–4168 (1999).
Davies, S. J. et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683 (1997).
Davies, S. J., Goucher, D. R., Doller, C. & Silver, J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19, 5810–5822 (1999).
Lehmann, M. et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19, 7537–7547 (1999).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Jin, Z. & Strittmatter, S. M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 17, 6256–6263 (1997).
Fournier, A. E., Takizawa, B. T. & Strittmatter, S. M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23, 1416–1423 (2003).
Niederost, B., Oertle, T., Fritsche, J., McKinney, R. A. & Bandtlow, C. E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376 (2002).
Vinson, M. et al. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J. Biol. Chem. 276, 20280–20285 (2001).
Yamashita, T. & Tohyama, M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nature Neurosci. 6, 461–467 (2003).
Dergham, P. et al. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22, 6570–6577 (2002).
Igarashi, M., Strittmatter, S. M., Vartanian, T. & Fishman, M. C. Mediation by G proteins of signals that cause collapse of growth cones. Science 259, 77–79 (1993).
Cai, D., Shen, Y., de Bellard, M., Tang, S. & Filbin, M. T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22, 89–101 (1999).
Bandtlow, C. E., Schmidt, M. F., Hassinger, T. D., Schwab, M. E. & Kater, S. B. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 259, 80–83 (1993).
Hempstead, B. L. The many faces of p75NTR. Curr. Opin. Neurobiol. 12, 260–267 (2002).
Fournier, A. E., Gould, G. C., Liu, B. P. & Strittmatter, S. M. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J. Neurosci. 22, 8876–8883 (2002).
Winton, M. J., Dubreuil, C. I., Lasko, D., Leclerc, N. & McKerracher, L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 277, 32820–32829 (2002).
Bandtlow, C. E. Regeneration in the central nervous system. Exp. Gerontol. 38, 79–86 (2003).
Song, H. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518 (1998).
Ramer, M. S., Priestley, J. V. & McMahon, S. B. Functional regeneration of sensory axons into the adult spinal cord. Nature 403, 312–316 (2000).
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002). This study, as well as reference 96, demonstrates for the first time that elevation of cAMP in vivo , at the cell body, can induce spinal axon regeneration.
Cai, D. et al. Arginase I and polyamines are downstream from cyclic AMP in the pathway that overcomes inhibition of axonal regeneration by Mag and myelin in vitro. Neuron 35, 711–719 (2002).
Chu, P. J., Saito, H. & Abe, K. Polyamines promote regeneration of injured axons of cultured rat hippocampal neurons. Brain Res. 673, 233–241 (1995).
Dornay, M., Gilad, V. H., Shiler, I. & Gilad, G. M. Early polyamine treatment accelerates regeneration of rat sympathetic neurons. Exp. Neurol. 92, 665–674 (1986).
Kauppila, T., Stenberg, D. & Porkka-Heiskanen, T. Putative stimulants for functional recovery after neural trauma: only spermine was effective. Exp. Neurol. 99, 50–58 (1988).
Banan, A., McCormack, S. A. & Johnson, L. R. Polyamines are required for microtubule formation during gastric mucosal healing. Am. J. Physiol. 274, G879–885 (1998).
Kaminska, B., Kaczmarek, L. & Grzelakowska-Sztabert, B. Inhibitors of polyamine biosynthesis affect the expression of genes encoding cytoskeletal proteins. FEBS Lett. 304, 198–200 (1992).
Wolff, J. Promotion of microtubule assembly by oligocations: cooperativity between charged groups. Biochemistry 37, 10722–10729 (1998).
Williams, K. Interactions of polyamines with ion channels. Biochem. J. 325, 289–297 (1997).
Chao, J. et al. N1-dansyl-spermine and N1-(n-octanesulfonyl)-spermine, novel glutamate receptor antagonists: block and permeation of N-methyl-D-aspartate receptors. Mol. Pharmacol. 51, 861–871 (1997).
Kashiwagi, K., Pahk, A. J., Masuko, T., Igarashi, K. & Williams, K. Block and modulation of N-methyl-D-aspartate receptors by polyamines and protons: role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Mol. Pharmacol. 52, 701–713 (1997).
Williams, K., Zappia, A. M., Pritchett, D. B., Shen, Y. M. & Molinoff, P. B. Sensitivity of the N-methyl-D–aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol. 45, 803–809 (1994).
Cohen, S. (ed.) A Guide to the Polyamines (Oxford Univ. Press, New York, 1998).
Aizenman, C. D., Munoz-Elias, G. & Cline, H. T. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34, 623–634 (2002).
Ming, G., Henley, J., Tessier-Lavigne, M., Song, H. & Poo, M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452 (2001).
Bregman, B. S. & Goldberger, M. E. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science 217, 553–555 (1982).
Kunkel-Bagden, E., Dai, H. N. & Bregman, B. S. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp. Neurol. 116, 40–51 (1992).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999). Shows that dorsal column axons will regenerate after a peripheral conditioning lesion without a peripheral nerve graft at the lesion site.
Richardson, P. M. & Issa, V. M. Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309, 791–793 (1984).
DeBellard, M. E., Tang, S., Mukhopadhyay, G., Shen, Y. J. & Filbin, M. T. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. Cell. Neurosci. 7, 89–101 (1996).
Neumann, S., Bradke, F., Tessier-Lavigne, M. & Basbaum, A. I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34, 885–893 (2002).
Bonilla, I. E., Tanabe, K. & Strittmatter, S. M. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 22, 1303–1315 (2002).
Schreyer, D. J. & Skene, J. H. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J. Neurosci. 11, 3738–3751 (1991).
Schreyer, D. J. & Skene, J. H. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J. Neurobiol. 24, 959–970 (1993).
Bomze, H. M., Bulsara, K. R., Iskandar, B. J., Caroni, P. & Skene, J. H. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci. 4, 38–43 (2001).
Scherer, S. S. & Salzer, J. L. in Glial Cell Development; Basic Principles and Clinical Relevance (eds Jessen, K. R. & Richardson, W. D.) 165–185 (Bios Scientific Publishers, Oxford, 1996).
Rapalino, O. et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature Med. 4, 814–821 (1998).
Leskovar, A., Moriarty, L. J., Turek, J. J., Schoenlein, I. A. & Borgens, R. B. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. J. Exp. Biol. 203, 1783–1795 (2000).
Kelm, S. et al. Functional groups of sialic acids involved in binding to siglecs (sialoadhesins) deduced from interactions with synthetic analogues. Eur. J. Biochem. 255, 663–672 (1998).
Collins, B. E. et al. Sialic acid specificity of myelin-associated glycoprotein binding. J. Biol. Chem. 272, 1248–1255 (1997).
Vyas, A. A. et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc. Natl Acad. Sci. USA 99, 8412–8417 (2002).
Yang, L. J. et al. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl Acad. Sci. USA 93, 814–818 (1996).
Bandtlow, C. E. & Loschinger, J. Developmental changes in neuronal responsiveness to the CNS myelin-associated neurite growth inhibitor NI-35/250. Eur. J. Neurosci. 9, 2743–2752 (1997).
Acknowledgements
This review is dedicated to the memory of N. Kimura. I thank R. Persell for critically reading this manuscript and T. Spencer for creating the figures. Funding was provided by the NIH/NINDS/RCMI/SNRP, the National Multiple Sclerosis Society, USA and the New York State Spinal Cord Initiative.
Author information
Authors and Affiliations
Glossary
- LECTINS
-
Sugar-binding proteins that tend to agglutinate cells.
- PARANODAL LOOPS
-
Concertina-shaped folds of myelin, which flank the nodes of Ranvier.
- SCHMIDT-LANTERMAN INCISURES
-
Funnel-shaped interruptions in the myelin sheath that were shown by electron microscopy to correspond to strands of cytoplasm separating two otherwise fused myelinating cell membranes.
- GLYCOSYL PHOSPHATIDYLINOSITOL
-
A post-translational modification, the function of which is to attach proteins to the exoplasmic leaflet of membranes, possibly to specific domains therein. The anchor is made of one molecule of phosphatidylinositol to which a carbohydrate chain is linked through the C6 hydroxyl of the inositol, and is attached to the protein through an ethanolamine phosphate moiety.
- IGM ANTIBODY
-
An immunoglobulin molecule that consists of five immunoglobulin G-type monomers, joined together by J chains to form a cyclic pentamer. During an immune response, IgM is usually produced before IgG, and forms the first line of defence.
- NODE OF RANVIER
-
A region of exposed plasma membrane in a myelinated axon. The nodes propagate action potentials by saltatory conduction, and they contain high concentrations of voltage-gated ion channels.
- DOMINANT NEGATIVE
-
A mutant molecule that can interfere with the normal molecule, knocking out its activity
- GANGLIOSIDE
-
An anionic glycosphingolipid that carries one or more sialic acid residues, in addition to other sugar residues.
- GENE TRAPPING
-
A mutation strategy that makes use of insertion vectors to trap or isolate transcripts from flanking genes. The inserted sequence acts as a tag from which to clone the mutated gene.
- DORSAL ROOT GANGLION
-
The cell bodies of sensory neurons are collected together in paired ganglia that lie alongside the spinal cord. These cell bodies are surrounded by satellite glial cells, which have much in common with the Schwann cells that ensheath peripheral axons. Very few synapses have been observed in these ganglia.
- SMALL GTPASE PROTEINS
-
A family of proteins that can hydrolyse GTP, which includes Rac, Rab, Ran, Rad, Rho and others. They subserve multiple cellular functions; for example, Rho and Rac are involved in the control of the cytoskeleton.
- PERTUSSIS TOXIN
-
The causative agent of whooping cough, pertussis toxin causes the persistent inactivation of Gi proteins by catalysing the ADP-ribosylation of the α-subunit.
- POLYAMINES
-
Organic compounds that contain two or more amino groups. Putrescine, spermine and spermidine are prime examples.
- INWARDLY RECTIFYING K+ CHANNELS
-
Potassium channels that allow long depolarizing responses, as they close during depolarizing pulses and open with steep voltage dependence on hyperpolarization. They are called inward rectifiers because current flows through them more easily into than out of the cell.
Rights and permissions
About this article
Cite this article
Filbin, M. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 4, 703–713 (2003). https://doi.org/10.1038/nrn1195
Issue date:
DOI: https://doi.org/10.1038/nrn1195
This article is cited by
-
CD36 deletion prevents white matter injury by modulating microglia polarization through the Traf5-MAPK signal pathway
Journal of Neuroinflammation (2024)
-
RhoGDI phosphorylation by PKC promotes its interaction with death receptor p75NTR to gate axon growth and neuron survival
EMBO Reports (2024)
-
NgR1 is an NK cell inhibitory receptor that destabilizes the immunological synapse
Nature Immunology (2023)
-
Regulation of axonal regeneration after mammalian spinal cord injury
Nature Reviews Molecular Cell Biology (2023)
-
Inhibition of the Nogo-pathway in experimental spinal cord injury: a meta-analysis of 76 experimental treatments
Scientific Reports (2023)