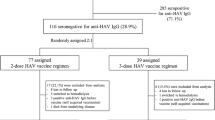
Key Points
-
In nephrology and transplant medicine, many opportunities for vaccination and protection against infection are missed
-
As immunogenicity is generally greater before transplantation, early in the course of renal disease—or at least before transplantation—is the optimal window of opportunity for vaccination
-
Delaying vaccination for the first 6–12 months after transplantation (or repeating vaccines given early) might result in higher rates of protection; nonetheless, influenza vaccine should be given in season
-
The reported impact of individual immunosuppressive agents on vaccine responses varies between studies; overall, a lower immunosuppressive protocol is more likely to result in clinical protection
-
Although some concern about increased HLA sensitization after vaccination exists, clinical data does not suggest harm; non-live vaccines appear immunogenic, protective and safe
-
Future research is needed into the impact of immunosuppressive protocols on vaccination responses, optimal timing after transplantation, dosing regimens, intradermal administration, clinical protection, use of adjuvants, safety and adverse effects
Abstract
Many transplant recipients are not protected against vaccine-preventable illnesses, primarily because vaccination is still an underutilized tool both before and after transplantation. This missed opportunity for protection can result in substantial morbidity, graft loss and mortality. Immunization strategies should be formulated early in the course of renal disease to maximize the likelihood of vaccine-induced immunity, particularly as booster or secondary antibody responses are less affected by immune compromise than are primary or de novo antibody responses in naive vaccine recipients. However, live vaccines should be avoided in immunocompromised hosts. Although some concern has been raised regarding increased HLA sensitization after vaccination, no clinical data to suggest harm currently exists; overall, non-live vaccines seem to be immunogenic, protective and safe. In organ transplant recipients, some vaccines are indicated based on specific risk factors and certain vaccines, such as hepatitis B, can protect against donor-derived infection. Vaccines given to close contacts of renal transplant recipients can provide an additional layer of protection against infectious diseases. In this article, optimal vaccination of adult transplant recipients, including safety, efficacy, indication and timing, is reviewed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bridges, C. B., Coyne-Beasley, T., Advisory Committee on Immunization Practices (ACIP), ACIP Adult Immunization Work Group & Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2014. MMWR Morb. Mortal. Wkly Rep. 63, 110–112 (2014).
World Health Organization. WHO recommendations for routine immunization—summary tables. Immunization, Vaccines and Biologicals [online], (2014).
Danziger-Isakov, L., Kumar, D. & AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am. J. Transplant. 13 (Suppl. 4), 311–317 (2013).
Rubin, L. G. et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 58, e44–e100 (2014).
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 9 (Suppl. 3), S1–S155 (2009).
Centers for Disease Control & Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2011. MMWR Morb. Mortal. Wkly Rep. 62, 66–72 (2013).
Struijk, G. H. et al. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am. J. Transplant. 13, 819–820 (2013).
Bond, T. C., Spaulding, A. C., Krisher, J. & McClellan, W. Mortality of dialysis patients according to influenza and pneumococcal vaccination status. Am. J. Kidney Dis. 60, 959–965 (2012).
Atkinson, W., Wolfe, S., & Hamborsky, J. (Eds) Epidemiology and Prevention of Vaccine-Preventable Diseases 12th edn (Public Health Foundation, 2012).
Harpaz, R., Ortega-Sanchez, I. R. & Seward, J. F. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 57, 1–30 (2008).
Marin, M., Guris, D., Chaves, S. S., Schmid, S. & Seward, J. F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 56, 1–40 (2007).
Kumar, D. et al. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am. J. Transplant. 10, 18–25 (2010).
Kumar, D. et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am. J. Transplant. 11, 2020–2030 (2011).
Siegrist, C. A. et al. Responses of solid organ transplant recipients to the AS03-adjuvanted pandemic influenza vaccine. Antivir. Ther. 17, 893–903 (2012).
Katerinis, I. et al. De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am. J. Transplant. 11, 1727–1733 (2011).
Danziger-Isakov, L. et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation 89, 838–844 (2010).
Baluch, A. et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am. J. Transplant. 13, 1026–1033 (2013).
Hurst, F. P., Lee, J. J., Jindal, R. M., Agodoa, L. Y. & Abbott, K. C. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin. J. Am. Soc. Nephrol. 6, 1192–1197 (2011).
Eckerle, I., Rosenberger, K. D., Zwahlen, M. & Junghanss, T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS ONE 8, e56974 (2013).
Kumar, D., Welsh, B., Siegal, D., Chen, M. H. & Humar, A. Immunogenicity of pneumococcal vaccine in renal transplant recipients—three year follow-up of a randomized trial. Am. J. Transplant. 7, 633–638 (2007).
Birdwell, K. A. et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am. J. Kidney Dis. 54, 112–121 (2009).
Blumberg, E. A. et al. The immunogenicity of influenza virus vaccine in solid organ transplant recipients. Clin. Infect. Dis. 22, 295–302 (1996).
Lawal, A. et al. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am. J. Transplant. 4, 1805–1809 (2004).
Kumar, D. et al. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am. J. Transplant. 13, 2411–2417 (2013).
Chon, W. J. et al. Changing attitudes toward influenza vaccination in U.S. Kidney transplant programs over the past decade. Clin. J. Am. Soc. Nephrol. 5, 1637–1641 (2010).
Danzinger-Isakov, L. & Kumar, D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am. J. Transplant. 9 (Suppl. 4), S258–S262 (2009).
Cordero, E. et al. Therapy with m-TOR inhibitors decreases the response to the pandemic influenza A H1N1 vaccine in solid organ transplant recipients. Am. J. Transplant. 11, 2205–2213 (2011).
John, S. et al. Prophylaxis of hepatitis B infection in solid organ transplant recipients. Therap. Adv. Gastroenterol. 6, 309–319 (2013).
Fiorante, S., Lopez-Medrano, F., Ruiz-Contreras, J. & Aguado, J. M. Vaccination against Streptococcus pneumoniae in solid organ transplant recipients [Spanish]. Enferm. Infecc. Microbiol. Clin. 27, 589–592 (2009).
Centers for Disease Control & Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep. 61, 816–819 (2012).
Smith, K. J., Nowalk, M. P., Raymund, M. & Zimmerman, R. K. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine 31, 3950–3956 (2013).
McCashland, T. M., Preheim, L. C. & Gentry, M. J. Pneumococcal vaccine response in cirrhosis and liver transplantation. J. Infect. Dis. 181, 757–760 (2000).
Posfay-Barbe, K. M. et al. Varicella-zoster immunization in pediatric liver transplant recipients: safe and immunogenic. Am. J. Transplant. 12, 2974–2985 (2012).
Pittet, L. F. & Posfay-Barbe, K. M. Immunization in transplantation: review of the recent literature. Curr. Opin. Organ Transplant. 18, 543–548 (2013).
Centers for Disease Control & Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb. Mortal. Wkly Rep. 62, 574–576 (2013).
Zuckerman, R. A. & Limaye, A. P. Varicella zoster virus (VZV) and herpes simplex virus (HSV) in solid organ transplant patients. Am. J. Transplant. 13 (Suppl. 3), 55–66 (2013).
Oxman, M. N. et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352, 2271–2284 (2005).
Beil, S., Kreuzer, M. & Pape, L. Course of immunization titers after pediatric kidney transplantation and association with glomerular filtration rate and kidney function. Transplantation 94, e69–e71 (2012).
Avery, R. K. & Ljungman, P. Prophylactic measures in the solid-organ recipient before transplantation. Clin. Infect. Dis. 33 (Suppl. 1), S15–S21 (2001).
Duchini, A., Goss, J. A., Karpen, S. & Pockros, P. J. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin. Microbiol. Rev. 16, 357–364 (2003).
Molrine, D. C. & Hibberd, P. L. Vaccines for transplant recipients. Infect. Dis. Clin. North Am. 15, 273–305 (2001).
Kotton, C. N. & Freedman, D. O. in CDC Health Information for International Travel 2014, Ch. 8 (ed. Brunette, G. W.) 544–555 (Oxford University Press, 2014).
Hinten, F., Meeuwis, K. A., van Rossum, M. M. & de Hullu, J. A. HPV-related (pre)malignancies of the female anogenital tract in renal transplant recipients. Crit. Rev. Oncol. Hematol. 84, 161–180 (2012).
Stark, K. et al. Immunogenicity and safety of hepatitis A vaccine in liver and renal transplant recipients. J. Infect. Dis. 180, 2014–2017 (1999).
Gunther, M. et al. Rapid decline of antibodies after hepatitis A immunization in liver and renal transplant recipients. Transplantation 71, 477–479 (2001).
Vora, N. M. et al. Raccoon rabies virus variant transmission through solid organ transplantation. JAMA 310, 398–407 (2013).
Kotton, C. N. & Hibberd, P. L. Travel medicine and transplant tourism in solid organ transplantation. Am. J. Transplant. 13 (Suppl. 4), 337–347 (2013).
Azevedo, L. S. et al. Yellow fever vaccination in organ transplanted patients: is it safe? A multicenter study. Transpl. Infect. Dis. 14, 237–241 (2012).
Kengsakul, K., Sathirapongsasuti, K. & Punyagupta, S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J. Med. Assoc. Thai. 85, 131–134 (2002).
Slifka, M. K. et al. Antiviral immune response after live yellow fever vaccination of a kidney transplant recipient treated with IVIG. Transplantation 95, e59–e61 (2013).
Wyplosz, B. et al. Persistence of yellow fever vaccine-induced antibodies after solid organ transplantation. Am. J. Transplant. 13, 2458–2461 (2013).
Ali, T. et al. Detection of influenza antigen with rapid antibody-based tests after intranasal influenza vaccination (FluMist). Clin. Infect. Dis. 38, 760–762 (2004).
Chi, C. et al. Guidelines for vaccinating dialysis patients and patients with chronic kidney disease. Centers for Disease Control & Prevention [online] (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Kotton, C. Immunization after kidney transplantation—what is necessary and what is safe?. Nat Rev Nephrol 10, 555–562 (2014). https://doi.org/10.1038/nrneph.2014.122
Published:
Issue date:
DOI: https://doi.org/10.1038/nrneph.2014.122
This article is cited by
-
Vaccination in patients with kidney failure: lessons from COVID-19
Nature Reviews Nephrology (2022)
-
Pre-transplant donor HBV DNA+ and male recipient are independent risk factors for treatment failure in HBsAg+ donors to HBsAg- kidney transplant recipients
BMC Infectious Diseases (2021)
-
Live Virus Vaccines in Transplantation: Friend or Foe?
Current Infectious Disease Reports (2015)