Abstract
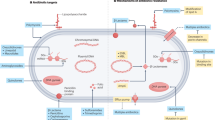
The urinary tract is a common site of bacterial infections; nearly half of all women experience at least one urinary tract infection (UTI) during their lifetime. These infections are classified based on the condition of the host. Uncomplicated infections affect otherwise healthy individuals and are most commonly caused by uropathogenic Escherichia coli, whereas complicated infections affect patients with underlying difficulties, such as a urinary tract abnormality or catheterization, and are commonly caused by species such as Proteus mirabilis. Virulence and fitness factors produced by both pathogens include fimbriae, toxins, flagella, iron acquisition systems, and proteins that function in immune evasion. Additional factors that contribute to infection include the formation of intracellular bacterial communities by E. coli and the production of urease by P. mirabilis, which can result in urinary stone formation. Innate immune responses are induced or mediated by pattern recognition receptors, antimicrobial peptides, and neutrophils. The adaptive immune response to UTI is less well understood. Host factors TLR4 and CXCR1 are implicated in disease outcome and susceptibility, respectively. Low levels of TLR4 are associated with asymptomatic bacteriuria while low levels of CXCR1 are associated with increased incidence of acute pyelonephritis. Current research is focused on the identification of additional virulence factors and therapeutic or prophylactic targets that might be used in the generation of vaccines against both uropathogens.
Key Points
-
The urinary tract is a common site of bacterial infection
-
Uropathogenic Escherichia coli and Proteus mirabilis are representative pathogens of uncomplicated and complicated urinary tract infection (UTI), respectively
-
Virulence and fitness factors synthesized by both pathogens include fimbriae, toxins, flagella, iron acquisition systems, and proteins that function in immune evasion
-
Additional factors that contribute to infection include the formation of intracellular bacterial communities by E. coli and the production of urease by P. mirabilis, which can result in urinary stone formation
-
Innate immune responses are induced or mediated by pattern recognition receptors, antimicrobial peptides, and neutrophils; the adaptive immune response to UTI is less well understood
-
Host factors TLR4 and CXCR1 are implicated in disease outcome and susceptibility, respectively; low levels of TLR4 are associated with asymptomatic bacteriuria; low levels of CXCR1 are associated with increased incidence of acute pyelonephritis
-
Reverse vaccinology approaches are currently underway to identify potential vaccine candidates for both pathogens
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Foxman, B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis. Mon. 49, 53–70 (2003).
Guay, D. R. Contemporary management of uncomplicated urinary tract infections. Drugs 68, 1169–1205 (2008).
O'Hanley, P. in Urinary Tract Infections: Molecular Pathogenesis and Clinical Management (eds Mobley, H. L. T. & Warren, J. W.) 405–425 (ASM Press, Washington, DC, 1996).
Zorc, J. J., Kiddoo, D. A. & Shaw, K. N. Diagnosis and management of pediatric urinary tract infections. Clin. Microbiol. Rev. 18, 417–422 (2005).
Litwin, M. S. & Saigal, C. S. (Eds) Urologic Diseases in America (Government Printing Office, Washington, D. C., 2007).
DeFrances, C. J., Lucas, C. A., Buie, V. C. & Golosinskiy, A. 2006 National Hospital Discharge Survey. National health statistics reports; no 5. (Hyattsville, MD, 2008).
Hooton, T. M. et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 50, 625–663 (2010).
Nicolle, L. E. Catheter-related urinary tract infection. Drugs Aging 22, 627–639 (2005).
Mobley, H. L., Donnenberg, M. S. & Hagan, E. C. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology (eds Böck, A. et al.) (ASM Press, Washington, DC, 2009).
Sosa, V., Schlapp, G. & Zunino, P. Proteus mirabilis isolates of different origins do not show correlation with virulence attributes and can colonize the urinary tract of mice. Microbiology 152, 2149–2157 (2006).
Welch, R. A. et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 99, 17020–17024 (2002).
Brzuszkiewicz, E. et al. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl Acad. Sci. USA 103, 12879–12884 (2006).
Chen, S. L. et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl Acad. Sci. USA 103, 5977–5982 (2006).
Touchon, M. et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5, e1000344 (2009).
Pearson, M. M. et al. The complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 190, 4027–4037 (2008).
Sabate, M., Moreno, E., Perez, T., Andreu, A. & Prats, G. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin. Microbiol. Infect. 12, 880–886 (2006).
Oelschlaeger, T. A., Dobrindt, U. & Hacker, J. Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int. J. Antimicrob. Agents 19, 517–521 (2002).
Ye, C. & Xu, J. Prevalence of iron transport gene on pathogenicity-associated island of uropathogenic Escherichia coli in E. coli O157:H7 containing Shiga toxin gene. J. Clin. Microbiol. 39, 2300–2305 (2001).
Lloyd, A. L., Rasko, D. A. & Mobley, H. L. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J. Bacteriol. 189, 3532–3546 (2007).
Flannery, E. L., Mody, L. & Mobley, H. L. Identification of a modular pathogenicity island that is widespread among urease-producing uropathogens and shares features with a diverse group of mobile elements. Infect. Immun. 77, 4887–4894 (2009).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Connell, I. et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl Acad. Sci. USA 93, 9827–9832 (1996).
Wu, X. R., Sun, T. T. & Medina, J. J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl Acad. Sci. USA 93, 9630–9635 (1996).
Lane, M. C. & Mobley, H. L. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 72, 19–25 (2007).
Westerlund, B. et al. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3, 329–337 (1989).
Goluszko, P. et al. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Invest. 99, 1662–1672 (1997).
Das, M. et al. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect. Immun. 73, 6119–6126 (2005).
Pere, A., Nowicki, B., Saxen, H., Siitonen, A. & Korhonen, T. K. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156, 567–574 (1987).
Buckles, E. L. et al. Identification and characterization of a novel uropathogenic Escherichia coli-associated fimbrial gene cluster. Infect. Immun. 72, 3890–3901 (2004).
Valle, J. et al. UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J. Bacteriol. 190, 4147–4161 (2008).
Boehm, D. F., Welch, R. A. & Snyder, I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect. Immun. 58, 1951–1958 (1990).
Island, M. D. et al. Cytotoxicity of hemolytic, cytotoxic necrotizing factor 1-positive and -negative Escherichia coli to human T24 bladder cells. Infect. Immun. 66, 3384–3389 (1998).
Mobley, H. L. et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58, 1281–1289 (1990).
Trifillis, A. L. et al. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 46, 1083–1091 (1994).
Uhlen, P. et al. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405, 694–697 (2000).
Smith, Y. C., Rasmussen, S. B., Grande, K. K., Conran, R. M. & O'Brien, A. D. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 76, 2978–2990 (2008).
O'Hanley, P., Lalonde, G. & Ji, G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect. Immun. 59, 1153–1161 (1991).
Boquet, P. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon 39, 1673–1680 (2001).
Lemonnier, M., Landraud, L. & Lemichez, E. Rho GTPase-activating bacterial toxins: from bacterial virulence regulation to eukaryotic cell biology. FEMS Microbiol. Rev. 31, 515–534 (2007).
Falzano, L. et al. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9, 1247–1254 (1993).
Hofman, P. et al. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68, 522–528 (2000).
Mills, M., Meysick, K. C. & O'Brien, A. D. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68, 5869–5880 (2000).
Johnson, D. E. et al. The role of cytotoxic necrotizing factor-1 in colonization and tissue injury in a murine model of urinary tract infection. FEMS Immunol. Med. Microbiol. 28, 37–41 (2000).
Rippere-Lampe, K. E., O'Brien, A. D., Conran, R. & Lockman, H. A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69, 3954–3964 (2001).
Parham, N. J. et al. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 230, 73–83 (2004).
Guyer, D. M., Radulovic, S., Jones, F. E. & Mobley, H. L. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70, 4539–4546 (2002).
Heimer, S. R., Rasko, D. A., Lockatell, C. V., Johnson, D. E. & Mobley, H. L. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72, 593–597 (2004).
Lane, M. C. et al. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73, 7644–7656 (2005).
Wright, K. J., Seed, P. C. & Hultgren, S. J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73, 7657–7668 (2005).
Snyder, J. A. et al. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72, 6373–6381 (2004).
Lane, M. C., Alteri, C. J., Smith, S. N. & Mobley, H. L. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl Acad. Sci. USA 104, 16669–16674 (2007).
Hagan, E. C. & Mobley, H. L. Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia coli for kidney infection. Mol. Microbiol. 71, 79–91 (2009).
Torres, A. G., Redford, P., Welch, R. A. & Payne, S. M. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69, 6179–6185 (2001).
Johnson, J. R. et al. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect. Immun. 73, 965–971 (2005).
Russo, T. A., Carlino, U. B. & Johnson, J. R. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69, 6209–6216 (2001).
Russo, T. A. et al. Iron functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70, 7156–7160 (2002).
Sabri, M., Houle, S. & Dozois, C. M. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect. Immun. 77, 1155–1164 (2009).
Goetz, D. H. et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 10, 1033–1043 (2002).
Fischbach, M. A. et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl Acad. Sci. USA 103, 16502–16507 (2006).
Smith, K. D. Iron metabolism at the host pathogen interface: lipocalin 2 and the pathogen-associated iroA gene cluster. Int. J. Biochem. Cell. Biol. 39, 1776–1780 (2007).
Cirl, C. et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14, 399–406 (2008).
Billips, B. K., Schaeffer, A. J. & Klumpp, D. J. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect. Immun. 76, 3891–3900 (2008).
Hunstad, D. A., Justice, S. S., Hung, C. S., Lauer, S. R. & Hultgren, S. J. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73, 3999–4006 (2005).
Johnson, J. R., Clabots, C. & Rosen, H. Effect of inactivation of the global oxidative stress regulator oxyR on the colonization ability of Escherichia coli O1:K1:H7 in a mouse model of ascending urinary tract infection. Infect. Immun. 74, 461–468 (2006).
Bower, J. M. & Mulvey, M. A. Polyamine-mediated resistance of uropathogenic Escherichia coli to nitrosative stress. J. Bacteriol. 188, 928–933 (2006).
Kulesus, R. R., Diaz-Perez, K., Slechta, E. S., Eto, D. S. & Mulvey, M. A. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect. Immun. 76, 3019–3026 (2008).
Svensson, L., Marklund, B. I., Poljakovic, M. & Persson, K. Uropathogenic Escherichia coli and tolerance to nitric oxide: the role of flavohemoglobin. J. Urol. 175, 749–753 (2006).
Lloyd, A. L., Smith, S. N., Eaton, K. A. & Mobley, H. L. Uropathogenic Escherichia coli suppresses the host inflammatory response via pathogenicity island genes sisA and sisB. Infect. Immun. 77, 5322–5333 (2009).
Li, K., Feito, M. J., Sacks, S. H. & Sheerin, N. S. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J. Immunol. 177, 2543–2551 (2006).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103, 14170–14175 (2006).
Anderson, G. G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107 (2003).
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 (2007).
Anderson, G. G., Martin, S. M. & Hultgren, S. J. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 6, 1094–1101 (2004).
Justice, S. S. et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl Acad. Sci. USA 101, 1333–1338 (2004).
Rocha, S. P., Pelayo, J. S. & Elias, W. P. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol. Med. Microbiol. 51, 1–7 (2007).
Bahrani, F. K., Johnson, D. E., Robbins, D. & Mobley, H. L. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect. Immun. 59, 3574–3580 (1991).
Jansen, A. M., Lockatell, V., Johnson, D. E. & Mobley, H. L. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect. Immun. 72, 7294–7305 (2004).
Li, X., Johnson, D. E. & Mobley, H. L. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67, 2822–2833 (1999).
Bahrani, F. K. et al. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62, 3363–3371 (1994).
Li, X., Lockatell, C. V., Johnson, D. E. & Mobley, H. L. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 45, 865–874 (2002).
Altman, E. et al. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell. Biol. 79, 783–788 (2001).
Lee, K. K., Harrison, B. A., Latta, R. & Altman, E. The binding of Proteus mirabilis nonagglutinating fimbriae to ganglio-series asialoglycolipids and lactosyl ceramide. Can. J. Microbiol. 46, 961–966 (2000).
Wray, S. K., Hull, S. I., Cook, R. G., Barrish, J. & Hull, R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect. Immun. 54, 43–49 (1986).
Massad, G., Lockatell, C. V., Johnson, D. E. & Mobley, H. L. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 62, 536–542 (1994).
Zunino, P. et al. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology 149, 3231–3237 (2003).
Massad, G., Bahrani, F. K. & Mobley, H. L. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 62, 1989–1994 (1994).
Zunino, P., Geymonat, L., Allen, A. G., Legnani-Fajardo, C. & Maskell, D. J. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol. Med. Microbiol. 29, 137–143 (2000).
Bijlsma, I. G., van Dijk, L., Kusters, J. G. & Gaastra, W. Nucleotide sequences of two fimbrial major subunit genes, pmpA and ucaA, from canine-uropathogenic Proteus mirabilis strains. Microbiology 141, 1349–1357 (1995).
Uphoff, T. S. & Welch, R. A. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172, 1206–1216 (1990).
Welch, R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect. Immun. 55, 2183–2190 (1987).
Alamuri, P. & Mobley, H. L. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68, 997–1017 (2008).
Alamuri, P., Eaton, K. A., Himpsl, S. D., Smith, S. N. & Mobley, H. L. Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect. Immun. 77, 632–641 (2009).
Rather, P. N. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7, 1065–1073 (2005).
Jansen, A. M., Lockatell, C. V., Johnson, D. E. & Mobley, H. L. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect. Immun. 71, 3607–3613 (2003).
Jones, B. V., Young, R., Mahenthiralingam, E. & Stickler, D. J. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect. Immun. 72, 3941–3950 (2004).
Mobley, H. L. et al. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 64, 5332–5340 (1996).
Zunino, P., Piccini, C. & Legnani-Fajardo, C. Flagellate and non-flagellate Proteus mirabilis in the development of experimental urinary tract infection. Microb. Pathog. 16, 379–385 (1994).
Evanylo, L. P., Kadis, S. & Maudsley, J. R. Siderophore production by Proteus mirabilis. Can. J. Microbiol. 30, 1046–1051 (1984).
Lima, A., Zunino, P., D'Alessandro, B. & Piccini, C. An iron-regulated outer-membrane protein of Proteus mirabilis is a haem receptor that plays an important role in urinary tract infection and in in vivo growth. J. Med. Microbiol. 56, 1600–1607 (2007).
Drechsel, H., Thieken, A., Reissbrodt, R., Jung, G. & Winkelmann, G. Alpha-keto acids are novel siderophores in the genera Proteus, Providencia, and Morganella and are produced by amino acid deaminases. J. Bacteriol. 175, 2727–2733 (1993).
Massad, G., Zhao, H. & Mobley, H. L. Proteus mirabilis amino acid deaminase: cloning, nucleotide sequence, and characterization of aad. J. Bacteriol. 177, 5878–5883 (1995).
Nielubowicz, G. R., Smith, S. N. & Mobley, H. L. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect. Immun. 78, 2823–2833 (2010).
Belas, R., Manos, J. & Suvanasuthi, R. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 72, 5159–5167 (2004).
Senior, B. W., Loomes, L. M. & Kerr, M. A. The production and activity in vivo of Proteus mirabilis IgA protease in infections of the urinary tract. J. Med. Microbiol. 35, 203–207 (1991).
Walker, K. E., Moghaddame-Jafari, S., Lockatell, C. V., Johnson, D. & Belas, R. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol. Microbiol. 32, 825–836 (1999).
Belas, R. & Flaherty, D. Sequence and genetic analysis of multiple flagellin-encoding genes from Proteus mirabilis. Gene 148, 33–41 (1994).
Belas, R. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J. Bacteriol. 176, 7169–7181 (1994).
Murphy, C. A. & Belas, R. Genomic rearrangements in the flagellin genes of Proteus mirabilis. Mol. Microbiol. 31, 679–690 (1999).
Zhao, H., Li, X., Johnson, D. E., Blomfield, I. & Mobley, H. L. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23, 1009–1019 (1997).
Li, X. et al. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect. Immun. 70, 389–394 (2002).
Thomas, W. E., Nilsson, L. M., Forero, M., Sokurenko, E. V. & Vogel, V. Shear-dependent 'stick-and-roll' adhesion of type 1 fimbriated Escherichia coli. Mol. Microbiol. 53, 1545–1557 (2004).
Wu, X. R., Kong, X. P., Pellicer, A., Kreibich, G. & Sun, T. T. Uroplakins in urothelial biology, function, and disease. Kidney Int. 75, 1153–1165 (2009).
Zhou, G. et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell. Sci. 114, 4095–4103 (2001).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Fukushi, Y., Orikasa, S. & Kagayama, M. An electron microscopic study of the interaction between vesical epitherlium and E. coli. Invest. Urol. 17, 61–68 (1979).
Klumpp, D. J. et al. Uropathogenic Escherichia coli potentiates type 1 pilus-induced apoptosis by suppressing NF-κB. Infect. Immun. 69, 6689–6695 (2001).
Sivick, K. E. & Mobley, H. L. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect. Immun. 78, 568–585 (2010).
Wullt, B. et al. The host response to urinary tract infection. Infect. Dis. Clin. North Am. 17, 279–301 (2003).
Chromek, M. et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641 (2006).
Morrison, G., Kilanowski, F., Davidson, D. & Dorin, J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060 (2002).
Valore, E. V. et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest. 101, 1633–1642 (1998).
Ganz, T. Iron in innate immunity: starve the invaders. Curr. Opin. Immunol. 21, 63–67 (2009).
Serafini-Cessi, F., Malagolini, N. & Cavallone, D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am. J. Kidney Dis. 42, 658–676 (2003).
Raffi, H. S., Bates, J. M., Jr, Laszik, Z. & Kumar, S. Tamm-horsfall protein protects against urinary tract infection by Proteus mirabilis. J. Urol. 181, 2332–2338 (2009).
Ingersoll, M. A., Kline, K. A., Nielsen, H. V. & Hultgren, S. J. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell. Microbiol. 10, 2568–2578 (2008).
Sivick, K. E., Schaller, M. A., Smith, S. N. & Mobley, H. L. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J. Immunol. 184, 2065–2075 (2010).
Hedges, S. et al. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62, 2315–2321 (1994).
Schilling, J. D., Mulvey, M. A., Vincent, C. D., Lorenz, R. G. & Hultgren, S. J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166, 1148–1155 (2001).
Wullt, B. et al. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell. Microbiol. 3, 255–264 (2001).
Agace, W. W. The role of the epithelial cell in Escherichia coli induced neutrophil migration into the urinary tract. Eur. Respir. J. 9, 1713–1728 (1996).
Agace, W. W., Hedges, S. R., Ceska, M. & Svanborg, C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Invest. 92, 780–785 (1993).
Hang, L. et al. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J. Immunol. 162, 3037–3044 (1999).
Haraoka, M. et al. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180, 1220–1229 (1999).
Ragnarsdottir, B. et al. TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur. J. Clin. Invest. 38 (Suppl. 2), 12–20 (2008).
Ashkar, A. A., Mossman, K. L., Coombes, B. K., Gyles, C. L. & Mackenzie, R. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog. 4, e1000233 (2008).
Schilling, J. D., Martin, S. M., Hung, C. S., Lorenz, R. G. & Hultgren, S. J. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 100, 4203–4208 (2003).
Patole, P. S. et al. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 68, 2582–2587 (2005).
Frendeus, B. et al. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 40, 37–51 (2001).
Hedges, S., Svensson, M. & Svanborg, C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect. Immun. 60, 1295–1301 (1992).
Hedlund, M. et al. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol. Microbiol. 33, 693–703 (1999).
Hedlund, M. et al. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39, 542–552 (2001).
Mossman, K. L. et al. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J. Immunol. 181, 6702–6706 (2008).
Fischer, H., Yamamoto, M., Akira, S., Beutler, B. & Svanborg, C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur. J. Immunol. 36, 267–277 (2006).
Andersen-Nissen, E. et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178, 4717–4720 (2007).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Thumbikat, P., Waltenbaugh, C., Schaeffer, A. J. & Klumpp, D. J. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 176, 3080–3086 (2006).
Pearsall, N. N. & Sherris, J. C. The demonstration of specific urinary anti-bodies in urinary tract infections caused by Gram-negative bacilli. J. Pathol. Bacteriol. 91, 589–595 (1966).
Eden, C. S., Hanson, L. A., Jodal, U., Lindberg, U. & Akerlund, A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet 1, 490–492 (1976).
Svanborg-Eden, C. & Svennerholm, A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect. Immun. 22, 790–797 (1978).
Trinchieri, A. et al. Secretory immunoglobulin A and inhibitory activity of bacterial adherence to epithelial cells in urine from patients with urinary tract infections. Urol. Res. 18, 305–308 (1990).
Svanborg, C. et al. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr. Opin. Microbiol. 9, 33–39 (2006).
Lomberg, H., Jodal, U., Eden, C. S., Leffler, H. & Samuelsson, B. P1 blood group and urinary tract infection. Lancet 1, 551–552 (1981).
Hagberg, L. et al. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46, 839–844 (1984).
Goluszko, P. et al. Vaccination with purified Dr Fimbriae reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect. Immun. 73, 627–631 (2005).
Ragnarsdottir, B. et al. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J. Infect. Dis. 196, 475–484 (2007).
Frendeus, B. et al. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J. Exp. Med. 192, 881–890 (2000).
Godaly, G., Hang, L., Frendeus, B. & Svanborg, C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J. Immunol. 165, 5287–5294 (2000).
Hang, L., Frendeus, B., Godaly, G. & Svanborg, C. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J. Infect. Dis. 182, 1738–1748 (2000).
Lundstedt, A. C. et al. A genetic basis of susceptibility to acute pyelonephritis. PLoS ONE 2, e825 (2007).
Lundstedt, A. C. et al. Inherited susceptibility to acute pyelonephritis: a family study of urinary tract infection. J. Infect. Dis. 195, 1227–1234 (2007).
Johnson, J. R. in Urinary Tract Infections: Molecular Pathogenesis and Clinical Management (eds Mobley, H. L. T. & Warren, J. W.) 95–118 (ASM Press, Washington, DC, 1996).
Svensson, M. et al. Glycolipid depletion in antimicrobial therapy. Mol. Microbiol. 47, 453–461 (2003).
Bishop, B. L. et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 13, 625–630 (2007).
Bouckaert, J. et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 55, 441–455 (2005).
Thankavel, K. et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J. Clin. Invest. 100, 1123–1136 (1997).
Lilly, J. D. & Parsons, C. L. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg. Gynecol. Obstet. 171, 493–496 (1990).
Maki, D. G. & Tambyah, P. A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7, 342–347 (2001).
Munasinghe, R. L., Yazdani, H., Siddique, M. & Hafeez, W. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect. Control Hosp. Epidemiol. 22, 647–649 (2001).
Stickler, D. J., Jones, G. L. & Russell, A. D. Control of encrustation and blockage of Foley catheters. Lancet 361, 1435–1437 (2003).
Hull, R. et al. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J. Urol. 163, 872–877 (2000).
Sabbuba, N. A. et al. Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J. Urol. 171, 1925–1928 (2004).
Bauer, H. W. et al. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur. Urol. 47, 542–548 (2005).
Hopkins, W. J., Elkahwaji, J., Beierle, L. M., Leverson, G. E. & Uehling, D. T. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J. Urol. 177, 1349–1353 (2007).
Sivick, K. E. & Mobley, H. L. An “omics” approach to uropathogenic Escherichia coli vaccinology. Trends Microbiol. 17, 431–432 (2009).
Alteri, C. J., Hagan, E. C., Sivick, K. E., Smith, S. N. & Mobley, H. L. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5, e1000586 (2009).
Johnson, D. E. et al. Serum immunoglobulin response and protection from homologous challenge by Proteus mirabilis in a mouse model of ascending urinary tract infection. Infect. Immun. 67, 6683–6687 (1999).
Li, X. & Mobley, H. L. Vaccines for Proteus mirabilis in urinary tract infection. Int. J. Antimicrob. Agents 19, 461–465 (2002).
Moayeri, N., Collins, C. M. & O'Hanley, P. Efficacy of a Proteus mirabilis outer membrane protein vaccine in preventing experimental Proteus pyelonephritis in a BALB/c mouse model. Infect. Immun. 59, 3778–3786 (1991).
Scavone, P. et al. Intranasal immunisation with recombinant Lactococcus lactis displaying either anchored or secreted forms of Proteus mirabilis MrpA fimbrial protein confers specific immune response and induces a significant reduction of kidney bacterial colonisation in mice. Microbes Infect. 9, 821–828 (2007).
Pellegrino, R., Galvalisi, U., Scavone, P., Sosa, V. & Zunino, P. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol. Med. Microbiol. 36, 103–110 (2003).
Li, X. et al. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect. Immun. 72, 7306–7310 (2004).
Nielubowicz, G. R., Smith, S. N. & Mobley, H. L. Outer membrane antigens of the uropathogen Proteus mirabilis recognized by the humoral response during experimental murine urinary tract infection. Infect. Immun. 76, 4222–4231 (2008).
Serruto, D., Serino, L., Masignani, V. & Pizza, M. Genome-based approaches to develop vaccines against bacterial pathogens. Vaccine 27, 3245–3250 (2009).
Abraham, J. M., Freitag, C. S., Clements, J. R. & Eisenstein, B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl Acad. Sci. USA 82, 5724–5727 (1985).
Klemm, P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5, 1389–1393 (1986).
Bryan, A. et al. Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073. Infect. Immun. 74, 1072–1083 (2006).
Lane, M. C., Li, X., Pearson, M. M., Simms, A. N. & Mobley, H. L. Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J. Bacteriol. 191, 1382–1392 (2009).
Li, X., Rasko, D. A., Lockatell, C. V., Johnson, D. E. & Mobley, H. L. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20, 4854–4862 (2001).
Pearson, M. M. & Mobley, H. L. Repression of motility during fimbrial expression: identification of 14 mrpJ gene paralogues in Proteus mirabilis. Mol. Microbiol. 69, 548–558 (2008).
Simms, A. N. & Mobley, H. L. PapX, a P fimbrial operon-encoded inhibitor of motility in uropathogenic Escherichia coli. Infect. Immun. 76, 4833–4841 (2008).
Acknowledgements
We sincerely apologize to authors whose important work could not be included in this article owing to space limitations. We would like to thank Erin Hagan, Melanie Pearson, Patrick Vigil, and Kelsey Sivick for thoughtful discussions. This work was supported in part by Public Health Service Grants AI43363 and AI059722 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nielubowicz, G., Mobley, H. Host–pathogen interactions in urinary tract infection. Nat Rev Urol 7, 430–441 (2010). https://doi.org/10.1038/nrurol.2010.101
Published:
Issue date:
DOI: https://doi.org/10.1038/nrurol.2010.101
This article is cited by
-
Uropathogen and host responses in pyelonephritis
Nature Reviews Nephrology (2023)
-
Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease
Nature Reviews Cardiology (2023)
-
Immunomodulation therapy offers new molecular strategies to treat UTI
Nature Reviews Urology (2022)
-
Targeting Microbial Bio-film: an Update on MDR Gram-Negative Bio-film Producers Causing Catheter-Associated Urinary Tract Infections
Applied Biochemistry and Biotechnology (2022)
-
Differences of virulence factors, and antimicrobial susceptibility according to phylogenetic group in uropathogenic Escherichia coli strains isolated from Korean patients
Annals of Clinical Microbiology and Antimicrobials (2021)