Abstract
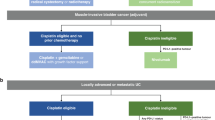
Upper tract urothelial cancer (UTUC) is an aggressive disease associated with significant morbidity and mortality. Radical nephroureterectomy (RNU) with bladder cuff removal is considered the standard of care for most invasive UTUCs but distant relapses after surgery for locally advanced, high-grade disease are common. Although multimodality treatment with perioperative chemotherapy has been investigated thoroughly in recent years, adjuvant chemotherapy has primarily been analyzed in small retrospective uncontrolled studies and a clear benefit for this treatment modality is yet to be established. It is likely that the high incidence of renal insufficiency after surgery substantially limits the applicability of adjuvant chemotherapy with cisplatin-based regimens. Neoadjuvant cisplatin-based chemotherapy has several practical advantages over adjuvant therapy, including better patient tolerance in the preoperative setting when a patient has two kidneys rather than one and the obtainment of prognostic information from pathological downstaging. Although, some academic centers have adopted neoadjuvant chemotherapy as a de facto treatment standard for patients with high-grade locally advanced UTUC, this treatment approach has not been prospectively validated or adopted in general urologic practice. A multicenter trial of neoadjuvant chemotherapy for locally advanced high-grade UTUC could further define the role of neoadjuvant chemotherapy in treating UTUC.
Key Points
-
Outcomes after surgery for patients with high-risk upper tract urothelial carcinoma (UTUC) are suboptimal
-
Chemotherapy has a role in the management of patients with advanced UTUC, including in the perioperative context
-
Decline in renal function after nephroureterectomy is common and restricts the use of cisplatin-based adjuvant chemotherapy regimens
-
Neoadjuvant chemotherapy has shown promising efficacy and excellent tolerability in preliminary datasets
-
Larger prospective trials are needed to further examine the utility of neoadjuvant chemotherapy treatment for UTUC
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Tawfiek, E. R. & Bagley, D. H. Upper-tract transitional cell carcinoma. Urology 50, 321–329 (1997).
Munoz, J. J. & Ellison, L. M. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J. Urol. 164, 1523–1525 (2000).
Konety, B. R., Joyce, G. F. & Wise, M. Bladder and upper tract urothelial cancer. J. Urol. 177, 1636–1645 (2007).
Lughezzani, G. et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur. J. Cancer 45, 3291–3297 (2009).
Margulis, V. et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115, 1224–1233 (2009).
Latini, D. et al. Upper tract urothelial cancer: a descriptive analysis using SEER data, 1973–2003. Presented at the Genitourinary Cancers Symposium 2008 (abstract no. 305).
Kaag, M. G. et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur. Urol. 58, 581–587 (2010).
Catto, J. W. et al. Multifocal urothelial cancers with the mutator phenotype are of monoclonal origin and require panurothelial treatment for tumor clearance. J. Urol. 175, 2323–2330 (2006).
Catto, J. W. et al. Behavior of urothelial carcinoma with respect to anatomical location. J. Urol. 177, 1715–1720 (2007).
Zhang, Z., Furge, K. A., Yang, X. J., Teh, B. T. & Hansel, D. E. Comparative gene expression profiling analysis of urothelial carcinoma of the renal pelvis and bladder. BMC Med. Genomics 3, 58 (2010).
Tsai, Y. S., Tzai, T. S., Chow, N. H. & Wu, C. L. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology 66, 1197–1202 (2005).
Eltz, S., Comperat, E., Cussenot, O. & Roupret, M. Molecular and histological markers in urothelial carcinomas of the upper urinary tract. BJU Int. 102, 532–535 (2008).
Hafner, C. et al. Evidence for oligoclonality and tumor spread by intraluminal seeding in multifocal urothelial carcinomas of the upper and lower urinary tract. Oncogene 20, 4910–4915 (2001).
Roscigno, M. et al. Assessment of the minimum number of lymph nodes needed to detect lymph node invasion at radical nephroureterectomy in patients with upper tract urothelial cancer. Urology 74, 1070–1074 (2009).
Roscigno, M. et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J. Urol. 181, 2482–2489 (2009).
Roscigno, M. et al. The extent of lymphadenectomy seems to be associated with better survival in patients with nonmetastatic upper-tract urothelial carcinoma: how many lymph nodes should be removed? Eur. Urol. 56, 512–518 (2009).
Keeley, F. X., Kulp, D. A., Bibbo, M., McCue, P. A. & Bagley, D. H. Diagnostic accuracy of ureteroscopic biopsy in upper tract transitional cell carcinoma. J. Urol. 157, 33–37 (1997).
Williams, S. K. et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J. Endourol. 22, 71–76 (2008).
Brown, G. A. et al. Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: implications for therapy. Urology 70, 252–256 (2007).
Skolarikos, A. et al. Cytologic analysis of ureteral washings is informative in patients with grade 2 upper tract TCC considering endoscopic treatment. Urology 61, 1146–1150 (2003).
Capitanio, U. et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur. Urol. 56, 1–9 (2009).
Favaretto, R. L. et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur. Urol. 58, 645–651 (2010).
McNeill, S. A., Chrisofos, M. & Tolley, D. A. The long-term outcome after laparoscopic nephroureterectomy: a comparison with open nephroureterectomy. BJU Int. 86, 619–623 (2000).
Rassweiler, J. J. et al. Laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: is it better than open surgery? Eur. Urol. 46, 690–697 (2004).
Simone, G. et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur. Urol. 56, 520–526 (2009).
Messer, J., Lin, Y. K. & Raman, J. D. The role of lymphadenectomy for upper tract urothelial carcinoma. Nat. Rev. Urol. 8, 394–401 (2011).
Karl, A. et al. The impact of lymphadenectomy and lymph node metastasis on the outcomes of radical cystectomy for bladder cancer. Eur. Urol. 55, 826–835 (2009).
Kirkali, Z. & Tuzel, E. Transitional cell carcinoma of the ureter and renal pelvis. Crit. Rev. Oncol. Hematol. 47, 155–169 (2003).
Brown, G. A. et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: Time to change the treatment paradigm? BJU Int. 98, 1176–1180 (2006).
Cozad, S. C. et al. Transitional cell carcinoma of the renal pelvis or ureter: patterns of failure. Urology 46, 796–800 (1995).
Cozad, S. C. et al. Adjuvant radiotherapy in high stage transitional cell carcinoma of the renal pelvis and ureter. Int. J. Radiat. Oncol. Biol. Phys. 24, 743–745 (1992).
Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361, 1927–1934 (2003).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
Tannock, I., Gospodarowicz, M., Connolly, J. & Jewett, M. MVAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J. Urol. 142, 289–292 (1989).
Lerner, S. E., Blute, M. L., Richardson, R. L. & Zincke, H. Platinum-based chemotherapy for advanced transitional cell carcinoma of the upper urinary tract. Mayo Clin. Proc. 71, 945–950 (1996).
Sternberg, C. N. et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J. Urol. 139, 461–469 (1988).
Loehrer, P. J. et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992).
Bamias, A. et al. Docetaxel and cisplatin with granulocyte colony-stimulating factor (G-CSF) versus MVAC with G-CSF in advanced urothelial carcinoma: a multicenter, randomized, phase III study from the Hellenic Cooperative Oncology Group. J. Clin. Oncol. 22, 220–228 (2004).
Kaufman, D. S. et al. A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urol. Oncol. 22, 393–397 (2004).
Czito, B., Zietman, A., Kaufman, D., Skowronski, U. & Shipley, W. Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J. Urol. 172, 1271–1275 (2004).
Kwak, C., Lee, S. E., Jeong I. G. & Ku, J. H. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology 68, 53–57 (2006).
Lee, S. E. et al. Adjuvant chemotherapy in the management of pT3N0M0 transitional cell carcinoma of the upper urinary tract. Urol. Int. 77, 22–26 (2006).
Soga, N., Arima, K. & Sugimura, Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int. J. Urol. 15, 800–803 (2008).
Hellenthal, N. J. et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J. Urol. 182, 900–906 (2009).
Vassilakopoulou, M. et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer 117, 5500–5508 (2011).
Bamias, A. et al. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J. Clin. Oncol. 22, 2150–2154 (2004).
Bellmunt, J. et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 80, 1966–1972 (1997).
Dreicer, R. et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer 100, 1639–1645 (2004).
Petrioli, R. et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer 77, 344–351 (1996).
Dogliotti, L. et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur. Urol. 52, 134–141 (2007).
Lane, B. R. et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 116, 2967–2973 (2010).
Ku, J. H., Choi, W. S., Kwak, C. & Kim, H. H. Bladder cancer after nephroureterectomy in patients with urothelial carcinoma of the upper urinary tract. Urol. Oncol. 29, 383–387 (2011).
Azemar, M. D., Comperat, E., Richard, F., Cussenot, O. & Roupret, M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol. Oncol. 29, 130–136 (2011).
Hall, M. C., Womack, J. S., Roehrborn, C. G., Carmody, T. & Sagalowsky, A. I. Advanced transitional cell carcinoma of the upper urinary tract: patterns of failure, survival and impact of postoperative adjuvant radiotherapy. J. Urol. 160, 703–706 (1998).
Hisataki, T. et al. Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology 55, 663–667 (2000).
Koda, S., Mita, K., Shigeta, M. & Usui, T. Risk factors for intravesical recurrence following urothelial carcinoma of the upper urinary tract: no relationship to the mode of surgery. Jpn J. Clin. Oncol. 37, 296–301 (2007).
Shikanov, S. et al. Vesical vs. extra-vesical patterns of recurrence after the treatment of urothelial upper tract tumors. Urol. Oncol. 26, 266–270 (2008).
Takaoka, E. et al. Pattern of intravesical recurrence after surgical treatment for urothelial cancer of the upper urinary tract: a single institutional retrospective long-term follow-up study. Int. J. Urol. 17, 623–628 (2010).
Zigeuner, R. E., Hutterer, G., Chromecki, T., Rehak, P. & Langner, C. Bladder tumor development after urothelial carcinoma of the upper urinary tract is related to primary tumor location. BJU Int. 98, 1181–1186 (2006).
Raman, J. D. et al. Bladder cancer after managing upper urinary tract transitional cell carcinoma: predictive factors and pathology. BJU Int. 96, 1031–1035 (2005).
Terakawa, T. et al. Risk factors for intravesical recurrence after surgical management of transitional cell carcinoma of the upper urinary tract. Urology 71, 123–127 (2008).
O'Brien, T. et al. Prevention of bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicenter, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C trial). Eur. Urol. 60, 703–710 (2011).
International collaboration of trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party et al. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet 354, 1650 (1999).
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–206 (2005).
Splinter, T. A., Denis, L., Scher, H. I., Schroder, F. H. & Dalesio, O. Neoadjuvant chemotherapy of invasive bladder cancer. The prognostic value of local tumor response. Prog. Clin. Biol. Res. 303, 541–547 (1989).
Matin, S. F. et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 116, 3127–3134 (2010).
Igawa, M. et al. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol. Int. 55, 74–77 (1995).
Siefker–Radtke, A. O. et al. A phase II trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine (IAG), followed by cisplatin, gemcitabine and ifosfamide (CGI) in locally advanced urothelial cancer (UC): final results from the M. D. Anderson Cancer Center. J. Clin. Oncol. 26, 5079 (2008).
Palapattu, G. S. et al. Cancer specific outcomes in patients with pT0 disease following radical cystectomy. J. Urol. 175, 1645–1649 (2006).
Tilki, D. et al. Stage pT0 at radical cystectomy confers improved survival: an international study of 4,430 patients. J. Urol. 184, 888–894 (2010).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Hall, M. C., Swanson, D. A. & Dinney, C. P. Complications of radical cystectomy: impact of the timing of perioperative chemotherapy. Urology 47, 826–830 (1996).
Millikan, R. et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J. Clin. Oncol. 19, 4005–4013 (2001).
Rajput, M. Z. et al. Perioperative outcomes of laparoscopic radical nephroureterectomy and regional lymphadenectomy in patients with upper urinary tract urothelial carcinoma after neoadjuvant chemotherapy. Urology 78, 68–71 (2011).
Sundi, D. et al. Upper tract urothelial carcinoma: Impact of time to surgery. Urol. Oncol. http://dx.doi.org/10.1016/j.urolonc.2010.04.002.
Gadzinski, A. J., Roberts, W. W., Faerber, G. J. & Wolf, J. S. Long-term outcomes of immediate versus delayed nephroureterectomy for upper tract urothelial carcinoma. J. Endourol. http://dx.doi.org/10.1089/end.2011.0220.
Vassilakopoulou, M. et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer 117, 5500–5508 (2011).
Hall, M. C. et al. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology 52, 594–601 (1998).
Shariat, S. F. et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int. 105, 1672–1677 (2010).
Cho, K. S., Hong, S. J., Cho, N. H. & Choi, Y. D. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology 70, 662–666 (2007).
Lehmann, J. et al. Transitional cell carcinoma of the ureter: prognostic factors influencing progression and survival. Eur. Urol. 51, 1281–1288 (2007).
Olgac, S., Mazumdar, M., Dalbagni, G. & Reuter, V. E. Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am. J. Surg. Pathol. 28, 1545–1552 (2004).
Simone, G. et al. Independent prognostic value of tumor diameter and tumor necrosis in upper urinary tract urothelial carcinoma. BJU Int. 103, 1052–1057 (2009).
Brien, J. C. et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J. Urol. 184, 69–73 (2010).
Ito, Y. et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J. Urol. 185, 1621–1626 (2011).
Ng, C. K. et al. Does the presence of hydronephrosis on preoperative axial CT imaging predict worse outcomes for patients undergoing nephroureterectomy for upper-tract urothelial carcinoma? Urol. Oncol. 29, 27–32 (2011).
Fritsche, H. M. et al. Macroscopic sessile tumor architecture is a pathologic feature of biologically aggressive upper tract urothelial carcinoma. Urol. Oncol. http://dx.doi.org/10.1016/j.urolonc.2010.07.010.
Remzi, M. et al. Tumor architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int. 103, 307–311 (2009).
Langner, C. et al. Tumor necrosis as prognostic indicator in transitional cell carcinoma of the upper urinary tract. J. Urol. 176, 910–914 (2006).
Seitz, C. et al. Association of tumor necrosis with pathological features and clinical outcome in 754 patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma: an international validation study. J. Urol. 184, 1895–1900 (2010).
Zigeuner, R. et al. Tumor necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur. Urol. 57, 575–581 (2010).
Guo, C. C., Tamboli, P. & Czerniak, B. Micropapillary variant of urothelial carcinoma in the upper urinary tract: a clinicopathologic study of 11 cases. Arch. Pathol. Lab. Med. 133, 62–66 (2009).
Holmang, S., Thomsen, J. & Johansson, S. L. Micropapillary carcinoma of the renal pelvis and ureter. J. Urol. 175, 463–467 (2006).
Perez–Montiel, D., Hes, O., Michal, M. & Suster, S. Micropapillary urothelial carcinoma of the upper urinary tract: clinicopathologic study of five cases. Am. J. Clin. Pathol. 126, 86–92 (2006).
Bolenz, C. et al. Lymphovascular invasion and pathologic tumor stage are significant outcome predictors for patients with upper tract urothelial carcinoma. Urology 72, 364–369 (2008).
Kikuchi, E. et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. 27, 612–618 (2009).
Kim, D. S. et al. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology 75, 328–332 (2010).
Novara, G. et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur. Urol. 57, 1064–1071 (2010).
Chromecki, T. F. et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 61, 245–253 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Author information
Authors and Affiliations
Contributions
A. Alva and A. O. Siefker-Radtke researched data and wrote the article. S. Matin and S. Lerner provided substantial contributions to discussions of content. All authors reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Alva, A., Matin, S., Lerner, S. et al. Perioperative chemotherapy for upper tract urothelial cancer. Nat Rev Urol 9, 266–273 (2012). https://doi.org/10.1038/nrurol.2012.57
Published:
Issue date:
DOI: https://doi.org/10.1038/nrurol.2012.57
This article is cited by
-
Expression of Estrogen Receptor Beta Predicts Oncologic Outcome of pT3 Upper Urinary Tract Urothelial Carcinoma Better Than Aggressive Pathological Features
Scientific Reports (2016)
-
Are urothelial carcinomas of the upper urinary tract a distinct entity from urothelial carcinomas of the urinary bladder? Behavior of urothelial carcinoma after radical surgery with respect to anatomical location: a case control study
BMC Cancer (2015)
-
Sequential chemotherapy using gemcitabine + carboplatin followed by gemcitabine + carboplatin + docetaxel for advanced upper-tract urothelial cancer
International Journal of Clinical Oncology (2015)
-
The Role of Systemic Chemotherapy in Management of Upper Tract Urothelial Cancer
Current Urology Reports (2013)