Abstract
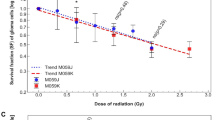
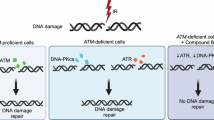
The phenomenon by which irradiated cells influence non-irradiated neighboring cells, referred to as the bystander effect (BSE), is not well understood in terms of the underlying pathways involved. We sought to enlighten connections between DNA damage repair and the BSE. Utilizing sister chromatid exchange (SCE) frequencies as a marker of the BSE, we performed cell transfer strategies that enabled us to distinguish between generation versus reception of a bystander signal. We find that DNA-dependent Protein Kinase catalytic subunit (DNA-PKcs) and Ataxia Telangectasia Mutated (ATM) are necessary for the generation of such a bystander signal in normal human cells following gamma (γ)-ray exposure, but are not required for its reception. Importantly, we also show that directly irradiated human cells do not respond to receipt of a bystander signal, helping to explain why the BSE is a low-dose phenomenon. These studies provide the first evidence for a role of the DNA damage response proteins DNA-PKcs and ATM specifically in the generation of a bystander signal and intercellular signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ardito G, Lamberti L, Piccotti F, Poggio G . (1980). Analysis of chromosome aberrations after exposure to ionizing radiation in ‘in vitro’ cultures of human lymphocytes by means of BrdU incorporation. Boll Soc Ital Biol Sper 56: 719–724.
Azzam EI, de Toledo SM, Gooding T, Little JB . (1998). Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res 150: 497–504.
Azzam EI, de Toledo SM, Little JB . (2001). Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 98: 473–478.
Bailey SM, Brenneman MA, Halbrook J, Nickoloff JA, Ullrich RL, Goodwin EH . (2004). The kinase activity of DNA-PK is required to protect mammalian telomeres. DNA Repair (Amst) 3: 225–233.
Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH . (2001). Strand-specific postreplicative processing of mammalian telomeres. Science 293: 2462–2465.
Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE et al. (1999). DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA 96: 14899–14904.
Banaz-Yasar F, Lennartz K, Winterhager E, Gellhaus A . (2007). Radiation-induced bystander effects in malignant trophoblast cells are independent from gap junctional communication. J Cell Biochem 103: 149–161.
Barzilai A, Rotman G, Shiloh Y . (2002). ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 1: 3–25.
Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP et al. (1996). Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA 93: 10285–10290.
Collis SJ, DeWeese TL, Jeggo PA, Parker AR . (2005). The life and death of DNA-PK. Oncogene 24: 949–961.
Denchi EL, de Lange T . (2007). Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448: 1068–1071.
Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE . (1996). Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: evidence for an extranuclear target. Radiat Res 145: 260–267.
Dudley DD, Chaudhuri J, Bassing CH, Alt FW . (2005). Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Adv Immunol 86: 43–112.
Fritz G, Kaina B . (2006). Late activation of stress kinases (SAPK/JNK) by genotoxins requires the DNA repair proteins DNA-PKcs and CSB. Mol Biol Cell 17: 851–861.
Grifalconi M, Celotti L, Mognato M . (2007). Bystander response in human lymphoblastoid TK6 cells. Mutat Res 625: 102–111.
Hu B, Wu L, Han W, Zhang L, Chen S, Xu A et al. (2006). The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis 27: 245–251.
Iyer R, Lehnert BE . (2002). Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res 157: 3–7.
Jackson SP, Jeggo PA . (1995). DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem Sci 20: 412–415.
Kanasugi Y, Hamada N, Wada S, Funayama T, Sakashita T, Kakizaki T et al. (2007). Role of DNA-PKcs in the bystander effect after low- or high-LET irradiation. Int J Radiat Biol 83: 73–80.
Kashino G, Prise KM, Suzuki K, Matsuda N, Kodama S, Suzuki M et al. (2007). Effective suppression of bystander effects by DMSO treatment of irradiated CHO cells. J Radiat Res (Tokyo) 48: 327–333.
Lavin MF, Kozlov S . (2007). ATM activation and DNA damage response. Cell Cycle 6: 931–942.
Lehnert BE, Goodwin EH, Deshpande A . (1997). Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res 57: 2164–2171.
Lieber MR . (1999). The biochemistry and biological significance of nonhomologous DNA end joining: an essential repair process in multicellular eukaryotes. Genes Cells 4: 77–85.
Meek K, Gupta S, Ramsden DA, Lees-Miller SP . (2004). The DNA-dependent protein kinase: the director at the end. Immunol Rev 200: 132–141.
Morgan WF . (2003). Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene 22: 7094–7099.
Mothersill C, Seymour CB . (1998). Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res 149: 256–262.
Mothersill C, Seymour RJ, Seymour CB . (2004). Bystander effects in repair-deficient cell lines. Radiat Res 161: 256–263.
Nagasawa H, Huo L, Little JB . (2003). Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int J Radiat Biol 79: 35–41.
Nagasawa H, Little JB . (1992). Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res 52: 6394–6396.
Nagasawa H, Little JB . (2002). Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat Res 508: 121–129.
Nagasawa H, Peng Y, Wilson PF, Lio YC, Chen DJ, Bedford JS et al. (2005). Role of homologous recombination in the alpha-particle-induced bystander effect for sister chromatid exchanges and chromosomal aberrations. Radiat Res 164: 141–147.
O'Neill-Mehlenbacher A, Kilemade M, Elliott A, Mothersill C, Seymour C . (2007). Comparison of direct and bystander effects induced by ionizing radiation in eight fish cell lines. Int J Radiat Biol 83: 593–602.
Okayasu R, Suetomi K, Yu Y, Silver A, Bedford JS, Cox R et al. (2000). A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res 60: 4342–4345.
Pandita TK . (2002). ATM function and telomere stability. Oncogene 21: 611–618.
Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L et al. (2004). ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol 24: 1823–1835.
Seymour CB, Mothersill C . (2000). Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res 153: 508–511.
Shao C, Lyng FM, Folkard M, Prise KM . (2006). Calcium fluxes modulate the radiation-induced bystander responses in targeted glioma and fibroblast cells. Radiat Res 166: 479–487.
Weil MM, Brown BW, Serachitopol DM . (1997). Genotype selection to rapidly breed congenic strains. Genetics 146: 1061–1069.
Weinstock DM, Jasin M . (2006). Alternative pathways for the repair of RAG-induced DNA breaks. Mol Cell Biol 26: 131–139.
Wolff SH, Perry P . (1975). Insights on chromosome structure from sister chromatid exchange ratios and the lack of both isolabelling and heterolabelling as determined by the FPG technique. Exp Cell Res 93: 23–30.
Wu LJ, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z et al. (1999). Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci USA 96: 4959–4964.
Xu W, Liu L, Smith GC, Charles G . (2000). Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat Cell Biol 2: 339–345.
Yang H, Anzenberg V, Held KD . (2007). The time dependence of bystander responses induced by iron-ion radiation in normal human skin fibroblasts. Radiat Res 168: 292–298.
Yu Y, Okayasu R, Weil MM, Silver A, McCarthy M, Zabriskie R et al. (2001). Elevated breast cancer risk in irradiated BALB/c mice associates with unique functional polymorphism of the Prkdc (DNA-dependent protein kinase catalytic subunit) gene. Cancer Res 61: 1820–1824.
Zhang Y, Zhou J, Cao X, Zhang Q, Lim CU, Ullrich RL et al. (2007). Partial deficiency of DNA-PKcs increases ionizing radiation-induced mutagenesis and telomere instability in human cells. Cancer Lett 250: 63–73.
Acknowledgements
We thank Dakim Gaines for technical assistance. Support for this research is gratefully acknowledged from the NIH/NCI, Grants CA-09236-30 (RLU) and CA-043322-20 (RLU and SMB), the US Department of Energy (DOE), Grant DE-FG02-01ER63239 (RLU and SMB), and from the National Aeronautics and Space Administration (NASA), Grant NNJ04HD83G (SMB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Hagelstrom, R., Askin, K., Williams, A. et al. DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene 27, 6761–6769 (2008). https://doi.org/10.1038/onc.2008.276
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2008.276
Keywords
This article is cited by
-
Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche
Cell Death & Disease (2020)
-
Radiation, inflammation and the immune response in cancer
Mammalian Genome (2018)