Abstract
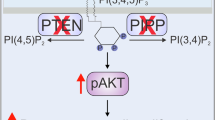
The ubiquitously expressed 14-3-3 proteins regulate many pathways involved in transformation. Previously, we found that 14-3-3ζ overexpression increased Akt phosphorylation in human mammary epithelial cells. Here, we investigated the clinical relevance and molecular mechanism of 14-3-3ζ-overexpression-mediated Akt phosphorylation, and its potential impact on breast cancer progression. We found that 14-3-3ζ overexpression was significantly (P=0.005) associated with increased Akt phosphorylation in human breast tumors. Additionally, 14-3-3ζ overexpression combined with strong Akt phosphorylation was significantly (P=0.01) associated with increased cancer recurrence in patients. In contrast, knockdown of 14-3-3ζ expression by small interfering RNA in cancer cell lines and tumor xenografts reduced Akt phosphorylation. Furthermore, 14-3-3ζ enhanced Akt phosphorylation through activation of phosphoinositide 3-kinase (PI3K). Mechanistically, 14-3-3ζ bound to the p85 regulatory subunit of PI3K and increased PI3K translocation to the cell membrane. A single 14-3-3-binding motif encompassing serine 83 on p85 is largely responsible for 14-3-3ζ-mediated p85 binding and PI3K/Akt activation. Mutation of serine 83 to alanine on p85 inhibited 14-3-3ζ binding to the p85 subunit of PI3K, reduced PI3K membrane localization and activation, impeded anchorage-independent growth and enhanced stress-induced apoptosis. These findings revealed a novel mechanism by which 14-3-3ζ overexpression activates PI3K, a key node in the mitogenic signaling network known to promote malignancies in many cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aitken A . (1995). 14-3-3 proteins on the MAP. Trends Biochem Sci 20: 90–97.
Aitken A . (2006). 14-3-3 proteins: a historic overview. Semin Cancer Biol 16: 162–172.
Barboric M, Kohoutek J, Price JP, Blazek D, Price DH, Peterlin BM . (2005). Interplay between 7SK snRNA and oppositely charged regions in HEXIM1 direct the inhibition of P-TEFb. Embo J 24: 4291–4303.
Benzinger A, Muster N, Koch HB, Yates III JR, Hermeking H . (2005). Targeted proteomic analysis of 14-3-3 sigma, a p53 effector commonly silenced in cancer. Mol Cell Proteomics 4: 785–795.
Bonnefoy-Berard N, Liu YC, von Willebrand M, Sung A, Elly C, Mustelin T et al. (1995). Inhibition of phosphatidylinositol 3-kinase activity by association with 14-3-3 proteins in T cells. Proc Natl Acad Sci USA 92: 10142–10146.
Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM et al. (2005). Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol Cell Biol 25: 2593–2606.
Cantley LC . (2002). The phosphoinositide 3-kinase pathway. Science 296: 1655–1657.
Cosentino C, Di Domenico M, Porcellini A, Cuozzo C, De Gregorio G, Santillo MR et al. (2007). p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 26: 2095–2103.
Craparo A, Freund R, Gustafson TA . (1997). 14-3-3 (epsilon) interacts with the insulin-like growth factor I receptor and insulin receptor substrate I in a phosphoserine-dependent manner. J Biol Chem 272: 11663–11669.
Danes CG, Wyszomierski SL, Lu J, Neal CL, Yang W, Yu D . (2008). 14-3-3 zeta down-regulates p53 in mammary epithelial cells and confers luminal filling. Cancer Res 68: 1760–1767.
De Gregorio G, Coppa A, Cosentino C, Ucci S, Messina S, Nicolussi A et al. (2007). The p85 regulatory subunit of PI3K mediates TSH-cAMP-PKA growth and survival signals. Oncogene 26: 2039–2047.
Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S et al. (2004). Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol 14: 1436–1450.
Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD . (2001). Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675.
Kim SJ, Cheon SH, Yoo SJ, Kwon J, Park JH, Kim CG et al. (2005). Contribution of the PI3K/Akt/PKB signal pathway to maintenance of self-renewal in human embryonic stem cells. FEBS Lett 579: 534–540.
Lonic A, Barry EF, Quach C, Kobe B, Saunders N, Guthridge MA . (2008). Fibroblast growth factor receptor 2 phosphorylation on serine 779 couples to 14-3-3 and regulates cell survival and proliferation. Mol Cell Biol 28: 3372–3385.
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B et al. (2009). 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16: 195–207.
Munday AD, Berndt MC, Mitchell CA . (2000). Phosphoinositide 3-kinase forms a complex with platelet membrane glycoprotein Ib-IX-V complex and 14-3-3zeta. Blood 96: 577–584.
Muslin AJ, Tanner JW, Allen PM, Shaw AS . (1996). Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84: 889–897.
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA et al. (2004). PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127.
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J et al. (2009). 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 69: 3425–3432.
Oksvold MP, Huitfeldt HS, Langdon WY . (2004). Identification of 14-3-3zeta as an EGF receptor interacting protein. FEBS Lett 569: 207–210.
Porter GW, Khuri FR, Fu H . (2006). Dynamic 14-3-3/client protein interactions integrate survival and apoptotic pathways. Semin Cancer Biol 16: 193–202.
Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N et al. (2004). 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J 379: 395–408.
Tan M, Yao J, Yu D . (1997). Overexpression of the c-erbB-2 gene enhanced intrinsic metastatic potential in human breast cancer cells without increasing their transformation abilities. Cancer Res 57: 1199–1205.
Tzivion G, Avruch J . (2002). 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem 277: 3061–3064.
Tzivion G, Gupta VS, Kaplun L, Balan V . (2006). 14-3-3 proteins as potential oncogenes. Semin Cancer Biol 16: 203–213.
Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC . (2005). Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30: 194–204.
van Hemert MJ, Steensma HY, van Heusden GP . (2001). 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays 23: 936–946.
Wilker E, Yaffe MB . (2004). 14-3-3 Proteins—a focus on cancer and human disease. J Mol Cell Cardiol 37: 633–642.
Yaffe MB . (2002). How do 14-3-3 proteins work?—Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett 513: 53–57.
Yaffe MB, Rittinger K, Volinia S, Caron PR, Aithen A, Jeffers H et al. (1997). The structural basis for 14-3-3: phosphopeptide binding specificity. Cell 91: 961–971.
Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM . (1998). Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol 18: 1379–1387.
Acknowledgements
This work is supported by grants from NIH P30-CA 16672 (MDACC); RO1-CA112567, PO1-CA099031 project 4, Department of Defense (DOD) Center of Excellence (BC050006) subproject, DOD Synergistic Award W81XWH-08-1-0712 and Susan G Komen Breast Cancer Foundation Promise Grant KG091020 (D Yu). Dr Dihua Yu is the Hubert L and Olive Stringer Distinguished Chair in Basic Science, MDACC. We thank Dr Lewis Cantley for providing p85 knockout MEFs. We also thank X Du, K Matias, T Chen, J Hannay, S Zhang and S Rehman for reagents, suggestions and manuscript reading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Neal, C., Xu, J., Li, P. et al. Overexpression of 14-3-3ζ in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 31, 897–906 (2012). https://doi.org/10.1038/onc.2011.284
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2011.284
Keywords
This article is cited by
-
Down-regulation of 14-3-3zeta reduces proliferation and increases apoptosis in human glioblastoma
Cancer Gene Therapy (2020)
-
Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells
Molecular and Cellular Biochemistry (2020)
-
Phosphorylation of 14-3-3ζ links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis
Oncogenesis (2019)
-
Myosin IIA Regulated Tight Junction in Oxygen Glucose-Deprived Brain Endothelial Cells Via Activation of TLR4/PI3K/Akt/JNK1/2/14-3-3ε/NF-κB/MMP9 Signal Transduction Pathway
Cellular and Molecular Neurobiology (2019)
-
Norepinephrine triggers an immediate-early regulatory network response in primary human white adipocytes
BMC Genomics (2018)