Abstract
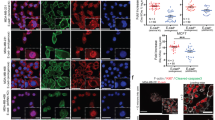
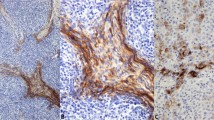
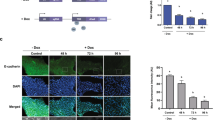
The genesis and unique properties of the lymphovascular tumor embolus are poorly understood largely because of the absence of an experimental model that specifically reflects this important step of tumor progression. The lymphovascular tumor embolus is a blastocyst-like structure resistant to chemotherapy, efficient at metastasis and overexpressing E-cadherin (E-cad). Conventional dogma has regarded E-cad as a metastasis-suppressor gene involved in epithelial–mesenchymal transition. However, within the lymphovascular embolus, E-cad and its proteolytic processing by calpain and other proteases have a dominant oncogenic rather than suppressive role in metastasis formation and tumor cell survival. Studies using a human xenograft model of inflammatory breast cancer, MARY-X, demonstrated the equivalence of xenograft-generated spheroids with lymphovascular emboli in vivo with both structures demonstrating E-cad overexpression and specific proteolytic processing. Western blot revealed full-length (FL) E-cad (120 kDa) and four fragments: E-cad/NTF1 (100 kDa), E-cad/NTF2 (95 kDa), E-cad/NTF3 (85 kDa) and E-cad/NTF4 (80 kDa). Compared with MARY-X, only E-cad/NTF1 was present in the spheroids. E-cad/NTF1 was produced by calpain, E-cad/NTF2 by γ-secretase and E-cad/NTF3 by a matrix metalloproteinase (MMP). Spheroidgenesis and lymphovascular emboli formation are the direct result of calpain-mediated cleavage of E-cad and the generation of E-cad/NTF1 from membrane-associated E-cad rather than the de novo presence of either E-cad/NTF1 or E-cad/CTF1. E-cad/NTF1 retained the p120ctn-binding site but lost both the β-catenin and α-binding sites, facilitating its disassembly from traditional cadherin-based adherens junctions and its 360° distribution around the embolus. This calpain-mediated proteolysis of E-cad generates the formation of the lymphovascular embolus and is responsible for its unique properties of increased homotypic adhesion, apoptosis resistance and budding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Berx G, Van Roy F . The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 2001; 3: 289–293.
Hajra KM, Chen DYS, Fearon ER . The slug zinc-finger protein suppresses E-cadherin in breast cancer. Cancer Res 2002; 62: 1613–1618.
Lombaerts M, van Wezel T, Philippo K, Dierssen JWF, Zimmerman RME, Oosting J et alE-cadherin transcriptional down-regulation by promoter methylation but not mutation is related to epithelial-to mesenchymal transition in breast cancer cell lines. Br J Cancer 2006; 94: 661–671.
Tomlinson JS, Alpaugh ML, Barsky SH . An intact overexpressed E-cadherin/α,β-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res 2001; 61: 5231–5241.
Albini A, Noonan DM . The ‘chemoinvasion’ assay, 25 years and still going strong: the use of reconstituted basement membranes to study cell invasion and angiogenesis. Curr Opin Cell Biol 2010; 22: 677–689.
Quigley JP, Armstrong PB . Tumor cell intravasation alu-cidated: the chick embryo opens the window. Cell 1998; 94: 281–284.
Nguyen M, Strubel NA, Bischoff J . A role for sialyl Lewis-X/A glycoconjugates in capillary morphogenesis. Nature 1993; 365: 267–269.
Shih C, Weinberg RA . Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell 1982; 29: 161–169.
Robertson FM, Ogasawara MA, Ye Z, Chu K, Pickei R, Debeb BG et al. Imaging and analysis of 3D tumor spheroids enriched for a cancer stem cell phenotype. J Biomol Screen 2010; 15: 820–829.
Ye Y, Tellez JD, Durazo M, Belcher M, Yearsley K, Barsky SH . E-cadherin accumulation within the lymphovascular embolus of inflammatory breast cancer is due to altered trafficking. Anticancer Res 2010; 10: 3903–3910.
Bryant D, Stow JL . The ins and outs of E-cadherin trafficking. Trends in Cell Biol 2004; 14: 427–434.
van IJzendoorn SC . Recycling endosomes. J Cell Sci 2006; 119: 1679–1681.
Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T . Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 2004; 279: 43027–43034.
Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U . Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol 2005; 169: 635–646.
Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 2005; 9: 365–376.
Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C . Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 2005; 25: 389–402.
Le Roy C, Wrana JL . Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 2005; 6: 112–126.
Kanazawa C, Morita E, Yamada M, Ishii N, Miura S, Asao H et al. Effects of deficiencies of STAMs and Hrs, mammalian class E Vps proteins, on receptor downregulation. Biochem Biophys Res Commun 2003; 309: 848–856.
Hammond DE, Carter S, McCullough J, Urbé S, Vande Woude G, Clague MJ . Endosomal dynamics of Met determine signaling output. Mol Biol Cell 2003; 14: 1346–1354.
Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M et al. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein hrs: its regulatory role on E-cadherin and β-catenin. Cancer Res 2007; 67: 5162–5171.
Feng Y, Press B, Wandinger-Ness A . Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol 1995; 131: 1435–1452.
Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB et al. Role of the small GTPase Rab 7 in the late endocytic pathway. J Biol Chem 1997; 272: 4391–4397.
Edinger AL, Cinalli RM, Thompson CB . Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev Cell 2003; 5: 571–582.
Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA et al. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem 2003; 278: 1372–1379.
Lebart MC, Benyamin Y . Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J 2006; 273: 3415–3426.
Franco SJ, Huttenlocher A . Regulating cell migration: calpains make the cut. J Cell Sci 2005; 118: 3829–3838.
Wells A, Huttenlocher A, Lauffenburger DA . Calpain proteases in cell adhesion and motility. Int Rev Cytol 2005; 245: 1–16.
Goll DE, Thompson VF, Li H, Wei W, Cong J . The calpain system. Physiol Rev 2003; 83: 731–801.
Chun J, Prince A . TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 2009; 5: 47–58.
Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene 1999; 18: 7080–7090.
Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 2002; 21: 1948–1956.
Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O . Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem 2001; 276: 4972–4980.
Herren B, Levkau B, Raines EW, Ross R . Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell 1998; 9: 1589–1601.
Gumbiner BM . Regulation of cadherin adhesive activity. J Cell Biol 2000; 148: 339–404.
Vallorosi CJ, Day KC, Zhao X, Rashid MG, Rubin MA, Johnson KR et al. Truncation of the beta-catenin binding of E-cadherin precedes epithelial apoptosis during prostate and mammary involution. J Biol Chem 2000; 113: 3328–3334.
Silvera D, Schneider RJ . Inflammatory breast cancer cells are constitutively adapted to hypoxia. Cell Cycle 2009; 8: 3091–3096.
Silvera D, Arju R, Darvishian F, Levine P, Zolfaghari L, Goldberg J et al. Essential role for elF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol 2009; 11: 903–908.
Chen X, Kojima S, Borisy GG, Green KJ . p120 catenin associates with kinesin and facilitates the transport of cadherin–catenin complexes to intercellular junctions. J Cell Biol 2003; 163: 547–557.
Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 2003; 163: 535–545.
Davis MA, Ireton RC, Reynolds AB . A core function for p120-catenin in cadherin turnover. J Cell Biol 2003; 163: 525–534.
Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thorenson MA et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol 2002; 159: 465–476.
Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382: 638–642.
Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H et al. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem 2008; 283: 12691–12700.
Alattia JR, Ames JB, Porumb T, Tong KI, Meng YH, Ottensmeyer P et al. Lateral self-assembly of E-cadherin directed by cooperative calcium binding. FEBS Lett 1997; 417: 405–408.
Koch AW, Pokutta S, Lustig A, Engel J . Calcium binding and homoassociation of E-cadherin domains. Biochemistry 1997; 36: 7697–7705.
Gopalakrishna R, Barsky SH . Quantitation of tissue calpain activity after isolation by hydrophobic chromatography. Anal Biochem 1985; 148: 413–423.
Gopalakrishna R, Barsky SH . Hydrophobic association of calpains with subcellular organelles—compartmentalization of calpains and the endogenous inhibitor calpastatin in tissues. J Biol Chem 1986; 261: 13936–13942.
Messaritou G, East L, Roghi C, Isacke CM, Yarwood H . Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180. J Cell Sci 2009; 122: 4042–4048.
Wakabayashi T, De Strooper B . Presenilins: members of the γ-secretase quartets, but part-time soloists too. Physiology 2008; 23: 194–204.
Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH . The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol 2008; 173: 561–574.
Xiao Y, Ye Y, Zou X, Jones S, Yearsley K, Shetuni B et al. The lymphovascular embolus of inflammatory breast cancer exhibits a Notch 3 addiction. Oncogene 2011; 30: 287–300.
Acknowledgements
We thank Dr John J Hasenau, Dr Walter F Mandeville, Patricia L Atkins and Jared H Smith of Laboratory Animal Medicine for their veterinarian and technical assistance with the maintenance of the MARY-X xenografts. This work was supported by the Department of Defense Breast Cancer Research Program Grants BC990959, BC024258 and BC053405, the American Airlines-Susan G Komen for the Cure Promise Grant KG081287-02 and the University of Nevada Vasco A Salvadorini Endowment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ye, Y., Tian, H., Lange, A. et al. The genesis and unique properties of the lymphovascular tumor embolus are because of calpain-regulated proteolysis of E-cadherin. Oncogene 32, 1702–1713 (2013). https://doi.org/10.1038/onc.2012.180
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2012.180
Keywords
This article is cited by
-
Geometric tumor embolic budding characterizes inflammatory breast cancer
Breast Cancer Research and Treatment (2023)
-
E-Cadherin fragments as potential mediators for peritoneal metastasis in advanced epithelial ovarian cancer
British Journal of Cancer (2016)
-
Gene/protein expression of CAPN1/2-CAST system members is associated with ERK1/2 kinases activity as well as progression and clinical outcome in human laryngeal cancer
Tumor Biology (2016)