Abstract
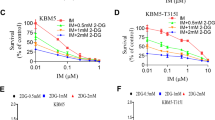
In chronic myelogenous leukemia, the constitutive activation of the BCR-ABL kinase transforms cells to an addicted state that requires glucose metabolism for survival. We investigated S6K1, a protein kinase that drives glycolysis in leukemia cells, as a target for counteracting glucose-dependent survival induced by BCR-ABL. BCR-ABL potently activated S6K1-dependent signaling and glycolysis. Although S6K1 knockdown or rapamycin treatment suppressed glycolysis in BCR-ABL-transformed cells, these treatments did not induce cell death. Instead, loss of S6K1 triggered compensatory activation of fatty-acid oxidation, a metabolic program that can support glucose-independent cell survival. Fatty-acid oxidation in response to S6K1 inactivation required the expression of the fatty-acid transporter carnitine palmitoyl transferase 1c, which was recently linked to rapamycin resistance in cancer. Finally, addition of an inhibitor of fatty-acid oxidation significantly enhanced cytotoxicity in response to S6K1 inactivation. These data indicate that S6K1 dictates the metabolic requirements mediating BCR-ABL survival and provide a rationale for combining targeted inhibitors of signal transduction, with strategies to interrupt oncogene-induced metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hsu PP, Sabatini DM . Cancer cell metabolism: warburg and beyond. Cell 2008; 134: 703–707.
Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB . Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem 2001; 276: 12041–12048.
Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB . Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 2003; 23: 7315–7328.
Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene 2005; 24: 4165–4173.
Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009; 461: 109–113.
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 2008; 105: 18782–18787.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007; 11: 37–51.
Vander Heiden MG . Targeting cell metabolism in cancer patients. Sci Transl Med 2010; 2: 31ed1.
Pelicano H, Martin DS, Xu RH, Huang P . Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25: 4633–4646.
Mason EF, Zhao Y, Goraksha-Hicks P, Coloff JL, Gannon H, Jones SN et al. Aerobic glycolysis suppresses p53 activity to provide selective protection from apoptosis upon loss of growth signals or inhibition of BCR-Abl. Cancer Res 2010; 70: 8066–8076.
Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ . Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res 2004; 10: 6661–6668.
Barnes K, McIntosh E, Whetton AD, Daley GQ, Bentley J, Baldwin SA . Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene 2005; 24: 3257–3267.
Klawitter J, Kominsky DJ, Brown JL, Christians U, Leibfritz D, Melo JV et al. Metabolic characteristics of imatinib resistance in chronic myeloid leukaemia cells. Br J Pharmacol 2009; 158: 588–600.
Kominsky DJ, Klawitter J, Brown JL, Boros LG, Melo JV, Eckhardt SG et al. Abnormalities in glucose uptake and metabolism in imatinib-resistant human BCR-ABL-positive cells. Clin Cancer Res 2009; 15: 3442–3450.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355: 2408–2417.
Branford S, Rudzki Z, Walsh S, Grigg A, Arthur C, Taylor K et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 2002; 99: 3472–3475.
Burchert A, Wang Y, Cai D, von Bubnoff N, Paschka P, Muller-Brusselbach S et al. Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia 2005; 19: 1774–1782.
Hochhaus A, Kreil S, Corbin AS, La Rosee P, Muller MC, Lahaye T et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002; 16: 2190–2196.
McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia 2008; 22: 708–722.
Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi JK et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J 1997; 16: 6151–6161.
Tandon P, Gallo CA, Khatri S, Barger JF, Yepiskoposyan H, Plas DR . Requirement for ribosomal protein S6 kinase 1 to mediate glycolysis and apoptosis resistance induced by Pten deficiency. Proc Natl Acad Sci USA 2011; 108: 2361–2365.
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 2009; 284: 8023–8032.
Markova B, Albers C, Breitenbuecher F, Melo JV, Brummendorf TH, Heidel F et al. Novel pathway in Bcr-Abl signal transduction involves Akt-independent, PLC-gamma1-driven activation of mTOR/p70S6-kinase pathway. Oncogene 2010; 29: 739–751.
Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB . Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol 2001; 21: 5899–5912.
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237–248.
Inoki K, Zhu T, Guan KL . TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590.
Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL . Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008; 10: 935–945.
Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 2005; 102: 14238–14243.
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008; 320: 1496–1501.
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006; 66: 1500–1508.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22: 159–168.
Nardella C, Lunardi A, Fedele G, Clohessy JG, Alimonti A, Kozma SC et al. Differential expression of S6K2 dictates tissue-specific requirement for S6K1 in mediating aberrant mTORC1 signaling and tumorigenesis. Cancer Res 2011; 71: 3669–3675.
Kharas MG, Janes MR, Scarfone VM, Lilly MB, Knight ZA, Shokat KM et al. Ablation of PI3K blocks BCR-ABL leukemogenesis in mice, and a dual PI3K/mTOR inhibitor prevents expansion of human BCR-ABL+ leukemia cells. J Clin Invest 2008; 118: 3038–3050.
Mohi MG, Boulton C, Gu TL, Sternberg DW, Neuberg D, Griffin JD et al. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci USA 2004; 101: 3130–3135.
Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell 2010; 38: 487–499.
Eaton S, Bartlett K, Pourfarzam M . Mammalian mitochondrial beta-oxidation. Biochem J 1996; 320 (Part 2): 345–357.
Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J . Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 2004; 25: 495–520.
Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev 2011; 25: 1041–1051.
Chalhoub N, Baker SJ . PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 2009; 4: 127–150.
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL et al. Activation of a metabolic gene regulatory network downstream of mTOR Complex 1. Mol Cell 2010; 39: 171–183.
Chalhoub N, Kozma SC, Baker SJ . S6k1 is not required for Pten-deficient neuronal hypertrophy. Brain Res 2006; 1100: 32–41.
Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010; 328: 1172–1176.
Um SH, D’Alessio D, Thomas G . Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 2006; 3: 393–402.
Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 2008; 453: 1072–1078.
Plas DR, Thomas G . Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol 2009; 21: 230–236.
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM . mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010; 468: 1100–1104.
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004; 431: 200–205.
Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 2011; 30: 2547–2557.
Djouadi F, Bonnefont JP, Munnich A, Bastin J . Characterization of fatty acid oxidation in human muscle mitochondria and myoblasts. Mol Genet Metab 2003; 78: 112–118.
Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC . Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 1998; 17: 6649–6659.
Acknowledgements
We thank Drs George Thomas and Sara Kozma for their advice and access to S6K1 knockout mice. We thank Dr Shane Horman, Jim Phelan and Meghan Brundage for their help with CML models in mice, and Drs Czyzyk-Krzeska and Godar for critique of the manuscript. JFB was supported by an IGERT fellowship #0333377. This work was supported by NIH Grant CA133164, the American Cancer Society RSG-08-293-01-CCG and the University of Cincinnati's Millennium Scholars program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Barger, J., Gallo, C., Tandon, P. et al. S6K1 determines the metabolic requirements for BCR-ABL survival. Oncogene 32, 453–461 (2013). https://doi.org/10.1038/onc.2012.70
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2012.70
Keywords
This article is cited by
-
Synergistic lethality in chronic myeloid leukemia – targeting oxidative phosphorylation and unfolded protein response effectively complements tyrosine kinase inhibitor treatment
BMC Cancer (2023)
-
Chronic myeloid leukemia stem cells and molecular target therapies for overcoming resistance and disease persistence
International Journal of Hematology (2018)
-
High mTORC1 activity drives glycolysis addiction and sensitivity to G6PD inhibition in acute myeloid leukemia cells
Leukemia (2017)
-
Pharmacological inhibition of fatty-acid oxidation synergistically enhances the effect of l-asparaginase in childhood ALL cells
Leukemia (2016)
-
Regulation of Hematopoietic Stem Cell Self-Renewal and Leukemia Maintenance by the PI3K-mTORC1 Pathway
Current Stem Cell Reports (2016)