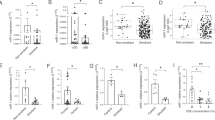
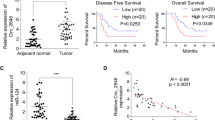
Abstract
Although microRNAs (miRs) have been implicated in the pathogenesis of various human malignancies, limited information is available regarding mechanisms by which these noncoding RNAs contribute to initiation and progression of tobacco-induced esophageal cancers. In this study, array and quantitative reverse transcriptase–PCR techniques were used to examine miR expression in immortalized esophageal epithelia (IEE) and esophageal adenocarcinoma (EAC) cells cultured in normal media with or without cigarette smoke condensate (CSC). Under relevant exposure conditions, CSC significantly decreased miR-217 expression in these cells. Endogenous levels of miR-217 expression in cultured EAC cells (EACC)/primary EACs were significantly lower than those observed in IEE/ paired normal esophageal tissues. RNA crosslink immunoprecipitation, quantitative reverse transcriptase–PCR (qRT–PCR) and immunoblot experiments demonstrated direct interaction of miR-217 with kallikrein 7 (KLK7), encoding a putative oncogene not previously implicated in EAC. Repression of miR-217 correlated with increased levels of KLK7 in primary EACs, particularly those from smokers. Chromatin and methylated DNA immunoprecipitation experiments demonstrated that CSC-mediated repression of miR-217 coincided with DNMT3b-dependent hypermethylation and decreased occupancy of nuclear factor 1 within the miR-217 genomic locus. Deoxyazacytidine induced miR-217 expression and downregulated KLK7 in EACC; deoxyazacytidine also attenuated CSC-mediated miR-217 repression and upregulation of KLK7 in IEE and EACC. Overexpression of miR-217 significantly decreased, whereas overexpression of KLK7 increased proliferation, invasion and tumorigenicity of EACC. Collectively, these data demonstrate that epigenetic repression of miR-217 contributes to the pathogenesis of EAC via upregulation of KLK7 and suggest that restoration of miR-217 expression may be a novel treatment strategy for these malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Agrawal N, Jiao Y, Bettegowda C, Hutfless SM, Wang Y, David S et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov 2012; 2: 899–905.
Dulak AM, Schumacher SE, van LJ, Imamura Y, Fox C, Shim B et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res 2012; 72: 4383–4393.
Napier KJ, Scheerer M, Misra S . Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6: 112–120.
Estores D, Velanovich V . Barrett esophagus: epidemiology, pathogenesis, diagnosis, and management. Curr Probl Surg 2013; 50: 192–226.
Spechler SJ . Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 2013; 310: 627–636.
Ek WE, Levine DM, D'Amato M, Pedersen NL, Magnusson PK, Bresso F et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett's esophagus, and gastroesophageal reflux. J Natl Cancer Inst 2013; 105: 1711–1718.
Carr JS, Zafar SF, Saba N, Khuri FR, El-Rayes BF . Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer 2013; 44: 143–151.
David S, Meltzer SJ . MicroRNA involvement in esophageal carcinogenesis. Curr Opin Pharmacol 2011; 11: 612–616.
Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res 2009; 15: 6192–6200.
Song JH, Meltzer SJ . MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology 2012; 143: 35–47.
Mayne GC, Hussey DJ, Watson DI . MicroRNAs and esophageal cancer—implications for pathogenesis and therapy. Curr Pharm Des 2013; 19: 1211–1226.
Fassan M, Volinia S, Palatini J, Pizzi M, Baffa R, De BM et al. MicroRNA expression profiling in human Barrett's carcinogenesis. Int J Cancer 2011; 129: 1661–1670.
Leidner RS, Ravi L, Leahy P, Chen Y, Bednarchik B, Streppel M et al. The microRNAs, MiR-31 and MiR-375, as candidate markers in Barrett's esophageal carcinogenesis. Genes Chromosomes Cancer 2012; 51: 473–479.
Devetzi M, Trangas T, Scorilas A, Xynopoulos D, Talieri M . Parallel overexpression and clinical significance of kallikrein-related peptidases 7 and 14 (KLK7KLK14) in colon cancer. Thromb Haemost 2013; 109: 716–725.
Dong Y, Tan OL, Loessner D, Stephens C, Walpole C, Boyle GM et al. Kallikrein-related peptidase 7 promotes multicellular aggregation via the alpha(5)beta(1) integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res 2010; 70: 2624–2633.
Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S et al. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest 2013; 123: 1241–1261.
Kent WJ . BLAT—the BLAST-like alignment tool. Genome Res 2002; 12: 656–664.
Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 2009; 11: 881–889.
Kemp CD, Rao M, Xi S, Inchauste S, Mani H, Fetsch P et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res 2012; 18: 77–90.
Rao M, Chinnasamy N, Hong JA, Zhang Y, Zhang M, Xi S et al. Inhibition of histone lysine methylation enhances cancer-testis antigen expression in lung cancer cells: implications for adoptive immunotherapy of cancer. Cancer Res 2011; 71: 4192–4204.
Diaz-Lopez A, Moreno-Bueno G, Cano A . Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag Res 2014; 6: 205–216.
Gu J, Xuan Z . Inferring the perturbed microRNA regulatory networks in cancer using hierarchical gene co-expression signatures. PLoS One 2013; 8: e81032.
Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL . The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PLoS One 2013; 8: e52192.
Tramacere I, La VC, Negri E . Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology 2011; 22: 344–349.
Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010; 102: 1344–1353.
Xu E, Gu J, Hawk ET, Wang KK, Lai M, Huang M et al. Genome-wide methylation analysis shows similar patterns in Barrett's esophagus and esophageal adenocarcinoma. Carcinogenesis 2013; 34: 2750–2756.
Frankel A, Armour N, Nancarrow D, Krause L, Hayward N, Lampe G et al. Genome-wide analysis of esophageal adenocarcinoma yields specific copy number aberrations that correlate with prognosis. Genes Chromosomes Cancer 2014; 53: 324–338.
Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J . The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis 2010; 31: 1726–1733.
Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci USA 2011; 108: 9863–9868.
Zhang Y, Xie RL, Gordon J, LeBlanc K, Stein JL, Lian JB et al. Control of mesenchymal lineage progression by microRNAs targeting skeletal gene regulators Trps1 and Runx2. J Biol Chem 2012; 287: 21926–21935.
Su J, Wang Q, Liu Y, Zhong M . miR-217 inhibits invasion of hepatocellular carcinoma cells through direct suppression of E2F3. Mol Cell Biochem 2014; 392: 289–296.
Nishioka C, Ikezoe T, Yang J, Nobumoto A, Tsuda M, Yokoyama A . Downregulation of miR-217 correlates with resistance of ph leukemia cells to ABL tyrosine kinase inhibitors. Cancer Sci 2014; 105: 297–307.
Shaw JL, Diamandis EP . Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem 2007; 53: 1423–1432.
Johnson SK, Ramani VC, Hennings L, Haun RS . Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer 2007; 109: 1811–1820.
Grabowska MM, Day ML . Soluble E-cadherin: more than a symptom of disease. Front Biosci (Landmark Ed) 2012; 17: 1948–1964.
Ramani VC, Haun RS . Expression of kallikrein 7 diminishes pancreatic cancer cell adhesion to vitronectin and enhances urokinase-type plasminogen activator receptor shedding. Pancreas 2008; 37: 399–404.
Ramani VC, Kaushal GP, Haun RS . Proteolytic action of kallikrein-related peptidase 7 produces unique active matrix metalloproteinase-9 lacking the C-terminal hemopexin domains. Biochim Biophys Acta 2011; 1813: 1525–1531.
Prezas P, Scorilas A, Yfanti C, Viktorov P, Agnanti N, Diamandis E et al. The role of human tissue kallikreins 7 and 8 in intracranial malignancies. Biol Chem 2006; 387: 1607–1612.
Mo L, Zhang J, Shi J, Xuan Q, Yang X, Qin M et al. Human kallikrein 7 induces epithelial-mesenchymal transition-like changes in prostate carcinoma cells: a role in prostate cancer invasion and progression. Anticancer Res 2010; 30: 3413–3420.
Prezas P, Arlt MJ, Viktorov P, Soosaipillai A, Holzscheiter L, Schmitt M et al. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7 increases the malignant phenotype of ovarian cancer cells. Biol Chem 2006; 387: 807–811.
Talieri M, Mathioudaki K, Prezas P, Alexopoulou DK, Diamandis EP, Xynopoulos D et al. Clinical significance of kallikrein-related peptidase 7 (KLK7) in colorectal cancer. Thromb Haemost 2009; 101: 741–747.
Iakovlev V, Siegel ER, Tsao MS, Haun RS . Expression of kallikrein-related peptidase 7 predicts poor prognosis in patients with unresectable pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2012; 21: 1135–1142.
Li H, Zhao J, Zhang JW, Huang QY, Huang JZ, Chi LS et al. MicroRNA-217, down-regulated in clear cell renal cell carcinoma and associated with lower survival, suppresses cell proliferation and migration. Neoplasma 2013; 60: 511–515.
Xi S, Yang M, Tao Y, Xu H, Shan J, Inchauste S et al. Cigarette smoke induces C/EBP-beta-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One 2010; 5: e13764.
Acknowledgements
We thank Ms Jan Pappas for assistance with manuscript preparation. This study was supported by NIH intramural funding and the Stephen J. Solarz Memorial Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Xi, S., Inchauste, S., Guo, H. et al. Cigarette smoke mediates epigenetic repression of miR-217 during esophageal adenocarcinogenesis. Oncogene 34, 5548–5559 (2015). https://doi.org/10.1038/onc.2015.10
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2015.10