Abstract
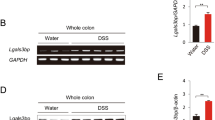
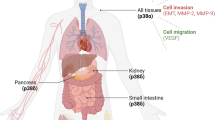
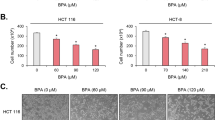
Chronic inflammation has long been considered to causatively link to colon cancer development. However, signal transduction pathways involved remain largely unidentified. Here, we report that p38γ mitogen-activated protein kinase mediates inflammatory signaling to promote colon tumorigenesis. Inflammation activates p38γ in mouse colon tissues and intestinal epithelial cell-specific p38γ knockout (KO) attenuates colitis and inhibits pro-inflammatory cytokine expression. Significantly, p38γ KO inhibits tumorigenesis in a colitis-associated mouse model. The specific p38γ pharmacological inhibitor pirfenidone also suppresses pro-inflammatory cytokine expression and colon tumorigenesis. The tumor-promoting activity of epithelial p38γ was further demonstrated by xenograft studies. In addition, p38γ is required for β-catenin/Wnt activities and p38γ stimulates Wnt transcription by phosphorylating β-catenin at Ser605. These results show that p38γ activation links inflammation and colon tumorigenesis. Targeting p38γ may be a novel strategy for colon cancer prevention and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Seril DD, Liao J, Yang GY, Yang CS . Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and anmial models. Carcinogenesis 2003; 24: 353–362.
Grivennikov S, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Kyriakis JM, Avruch J . Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 2012; 92: 689–737.
Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kB. Am J Pathol 2013; 182: 2310–2321.
Abdollahi T, Robertson NM, Abdollahi A, Litwack G . Inhibition of TRAIL-induced apoptosis by IL-8 is mediated by the p38-MAPK pathway in OVCAR3 cells. Apoptosis 2003; 10: 1383–1393.
Chen G, Hitomi M, Han J, Stacey DW . The p38 pathway provides negative feedback to Ras proliferative signaling. J Biol Chem 2000; 275: 38973–38980.
Qi X, Tang J, Pramanik R, Schultz RM, Shirasawa S, Sasazuki T et al. p38 MAPK activation selectively induces cell death in K-ras mutated human colon cancer cells through regulation of vitamin D receptor. J Biol Chem 2004; 279: 22138–22144.
Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CM et al. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16Ink4a-p19Arf pathway. Nat Genet 2004; 36: 343–350.
Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR . p38α MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 2007; 11: 191–205.
Hui L, Bakri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L et al. p38α suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet 2007; 39: 741–749.
Tamura T, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M . Requirement for p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 2000; 102: 221–231.
Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S et al. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 2000; 6: 109–116.
Otsuka M, Kang YJ, Ren J, Jiang H, Wang Y, Omata M et al. Distinct effects of p38α deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterol 2010; 138: 1255–1265.
Wakeman D, Schneider JE, Liu J, Wandu WS, Erwin CR, Guo J et al. Deletion of p38-alpha mitogen-activated protein kinase within the intestinal epithelium promotes colon tumorigenesis. Surgery 2012; 152: 286–293.
Gupta J, Barantes IB, Igea A, Sakellariou S, Pateras I, Gorgoulis VGea . Dual function of p38α MAPK in colon cancer: suppression of colities-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 2014; 25: 484–550.
Tang J, Qi X, Mercola D, Han J, Chen G . Essential role of p38γ in K-Ras transformation independent of phosphorylation. J Biol Chem 2005; 280: 23910–23917.
Qi X, Pohl NM, Loesch M, Hou S, Li R, Qin JZ et al. p38α antagonizes p38γ activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J Biol Chem 2007; 282: 31398–31408.
Qi X, Tang J, Loesch M, Pohl N, Alkan S, Chen G . p38γ MAPK integrates signaling cross-talk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Res 2006; 66: 7540–7547.
Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G . Differential tissue expression and activation of p38 MAPK α, β, γ, and δ isoforms in rheumatoid arthritis. Arthritis Rheumatism 2006; 54: 2745–2756.
Sabio G, Simon J, Arthur C, Kuma Y, Peggie M, Carr J et al. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J 2005; 24: 1134–1145.
Risco A, Fresno C, Mambol A, Alsina-Beauchamp D, MacKenzie KF, Yang H et al. p38γ and p38δ kinases regulate the toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc Natl Acad Sci USA 2012; 109: 11200–11205.
Hou SW, Zhi H, Pohl N, Loesch M, Qi X, Li R et al. PTPH1 dephosphorylates and cooperates with p38γ MAPK to increases Ras oncogenesis through PDZ-mediated interaction. Cancer Res 2010; 70: 2901–2910.
Qi XM, Xie C, Hou S, Li G, Yin N, Dong L et al. Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer. Oncotarget 2014; 5: 4269–4282.
Meng F, Zhang H, Liu G, Kreike B, Chen W, Sethi S et al. p38γ mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia 2011; 13: 472–482.
Rosenthal DT, Lyer H, Escudero S, Bao L, Wu Z, Ventura AC et al. p38γ promotes breast cancer motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior. Cancer Res 2011; 71: 6338–6349.
Qi X, Zhi H, Lepp A, Wang P, Huang J, Basir Z et al. p38γ mitogen-activated protein kinase (MAPK) confers breast cancer hormone sensitivity by switching estrogen receptor (ER) signaling from classical to nonclassical pathway via stimulating ER phosphorylation and c-Jun transcription. J Biol Chem 2012; 287: 14681–14691.
Liu L, Liu Z, Jiang H, Zhang W, Qi S, Hu J et al. Gene expression profiles of hepatoma cell line HLE. World J Gastroenterol 2003; 9: 683–687.
Yang K, Liu Y, Liu Z, Liu Z, Liu X, Chen X et al. p38γ overexpression in gliomas and its role in proliferation and apoptosis. Scientific Rep 2013; 3: 1–5.
Del Reino P, Alsina-Beauchamp D, Escos A, Cerezo-Guisado MI, Risco A, Aparicio N . Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38γ and p38δ, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res 2014; 74: 6150–6160.
Pogozelski A, Geng T, Li P, Lira V, Zhang M, Chi JT et al. p38γ mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. Plos One 2009; 4: e7934.
Rhodes JM, Campbell BL . Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med 2002; 8: 10–16.
Suzuki R, Kohno H, Nakagama H, Tanaka T . Strain differences in the susceptibility to azoxymethane and detran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis 2006; 27: 162–169.
Neufert C, Becker C, Neurath M . An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2007; 2: 1998–2004.
Loesch M, Zhi H, Hou S, Qi X, Li R, Basir Z et al. p38γ MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9. J Biol Chem 2010; 285: 15149–15158.
Moran N . p38 kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat Biotechnol 2011; 29: 301.
Noble PW, Albera C, Bradford W, Costabel U, Glassberg MK, Kardatzke D et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769.
Ozes O, Blatt LM, Seiwert SD Use of pirfenidone in therapeutic regimens. United States Patent-US 7,407,973 B2 2008; 5 August: 1-46.
Hou S, Padmanaban S, Qi X, Leep A, Mirza S, Chen G . p38γ MAPK signals through phosphorylating its phosphatase PTPH1 in regulating Ras oncogenesis and stress response. J Biol Chem 2012; 287: 27895–27905.
Clevers H . Wnt/β-catenin signaling in development and disease. Cell 2006; 127: 469–480.
Valenta T, Hausmann G, Basler K . The many faces and functions of β-catenin. EMBO J 2012; 31: 2714–2736.
Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A et al. The kinase p38α serves cell type-specific inflammatory functions in skin injury and cooridinates pro- and anti-inflammatory gene expression. Nat Immunol 2008; 9: 1019–1027.
Gillespie MA, Grand FL, Scime A, Kuang S, von Maltzahn J, Seale V et al. p38γ-dependent gene silencing restricts entry into the myogenic differentiation program. J Cell Biol 2009; 187: 991–1005.
Oku H, Nakazato H, Horikawa T, Suzuki R . Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotixic shock. Eur J Pharmacol 2002; 446: 167–176.
Schreiber S, Feagan B, D'Haens Gea . Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn' disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hapatol 2006; 4: 325–334.
Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F . Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell 2008; 133: 340–353.
Lee M, Koria P, Qu J, Andreadi ST . JNK phosphorylates β-catenin and regulates adherens junctions. FASEB J 2009; 23: 3874–3883.
Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S . β-Catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 2012; 18: 892–901.
Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res 2013; 73: 2345–2356.
Acknowledgements
We thank Boehringer Ingelheim Pharmaceuticals Inc. and Dr Zhen Yan (University of Virginia) for providing the floxed p38γ mice. We also thank former lab members Drs PS Suresh for maintaining mice and conducting some preliminary experiments, and S Hou for generating the p38γ-depleted HCT116 cell lines. In addition, we express our gratitude to Drs D Wang (Blood Institute of Wisconsin), A Chan, R Li and J Tichelaar (Medical College of Wisconsin) for useful discussions. We also thank Drs J Han (The Scripps Research Institute), A Cuenda (National Center for Biotechnology-CSIC), NK Tonks (Cold Spring Harbor) and H Howe (South Carolina University) for providing various reagents. This work was in part supported by the grants from the Department of Veterans Affairs Merit Review (I01BX002883), the Cancer Center of Medical College of Wisconsin (the State of Wisconsin Tax Check-Off Program), and Clinical & Translational Science Institute (CTSI) of Southeast Wisconsin (to GC).
Author Contributions
NY performed experiments, analyzed results and generated figures. XQ, GC, ZB and KO also participated in performing experiments. NY, XQ and GC designed experiments and interpreted results; GS, ST, JPT and CRM contributed ideas; and LY participated in data analysis. NY, CRM and GC wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Yin, N., Qi, X., Tsai, S. et al. p38γ MAPK is required for inflammation-associated colon tumorigenesis. Oncogene 35, 1039–1048 (2016). https://doi.org/10.1038/onc.2015.158
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2015.158
This article is cited by
-
G-quadruplex structural dynamics at MAPK12 promoter dictates transcriptional switch to determine stemness in breast cancer
Cellular and Molecular Life Sciences (2024)
-
P38 kinase in gastrointestinal cancers
Cancer Gene Therapy (2023)
-
ILC1 drive intestinal epithelial and matrix remodelling
Nature Materials (2021)
-
HMGB1 mediates lipopolysaccharide-induced inflammation via interacting with GPX4 in colon cancer cells
Cancer Cell International (2020)
-
Diclofenac induced apoptosis via altering PI3K/Akt/MAPK signaling axis in HCT 116 more efficiently compared to SW480 colon cancer cells
Molecular Biology Reports (2018)