Abstract
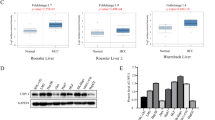
Downregulation of E-cadherin by the transcriptional repressor Snail is associated with acquisition of metastatic potential. Although hepatitis C virus (HCV) core protein has been implicated in hepatocarcinogenesis, it is unclear whether Snail is involved in HCV core-induced dysregulation of E-cadherin. Herein, we investigated the mechanism by which HCV core induces E-cadherin repression and the role of Snail in HCV core-mediated invasiveness and metastasis. We found that HCV infection, especially HCV core expression, effectively induced the epithelial–mesenchymal transition (EMT) in hepatoma cells by repressing E-cadherin. HCV core interacted with Snail and enhanced its binding to the E-box in the promoter region of E-cadherin, leading to decreased E-cadherin promoter activity. We found that HCV core, Snail, and the histone deacetylases HDAC1/HDAC2 formed a co-repressor complex at the E-cadherin promoter. Moreover, HCV core was shown to stabilize Snail through activation of the PI3K/Akt/GSK3β pathway. Silencing Snail expression restored E-cadherin expression and inhibited HCV core-promoted tumor growth and distant lung metastasis in vivo. Collectively, these results demonstrated that HCV core induced EMT by interacting with the transcriptional repressor complex Snail/HDACs at the E-cadherin promoter, which led to E-cadherin repression and increased invasiveness of hepatoma cells. These findings increase understanding of factors regulating metastasis in hepatoma and may ultimately lead to the development of novel treatment strategies for HCV-associated hepatocellular carcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA 1990; 87: 6547–6549.
Hugo R, Rosen MD . Chronic hepatitis C infection. N Engl J Med 2011; 364: 2429–2438.
Giannini C, Bréchot C . Hepatitis C virus biology. Cell Death Differ 2003; 10: S27–S38.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Hashiguchi M, Ueno S, Sakoda M, Iino S, Hiwatashi K, Minami K et al. Clinical implication of ZEB-1 and E-cadherin expression in hepatocellular carcinoma (HCC). BMC Cancer 2013; 13: 572.
Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2009; 101: 293–299.
Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84–89.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939.
Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA et al. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem 2001; 276: 27424–27431.
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ . Canonical Wnt signaling regulates Slug activity and links epithelial–mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci USA 2012; 109: 16654–16659.
Battaglia S, Benzoubir N, Nobilet S, Charneau P, Samuel D, Zignego AL et al. Liver cancer-derived hepatitis C virus core proteins shift TGF-beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS One 2009; 4: e4355.
Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial–mesenchymal transition. Oncogene 2014; 33: 2826–2835.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Garg M . Epithelial-mesenchymal transition-activating transcription factors— multifunctional regulators in cancer. World J. Stem Cells 2013; 5: 188–195.
Arzumanyan A, Friedman T, Kotei E, Ng IO, Lian Z, Feitelson MA . Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene 2012; 31: 563–572.
Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA . Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun 1997; 241: 453–458.
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2: 76–83.
Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F et al. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma Cells. PLoS One 2011; 6: e27496.
Bose SK, Meyer K, Di Bisceglie AM, Ray RB, Ray R . Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J Virol 2012; 86: 13621–13628.
Lindenbach BD, Rice CM . Unravelling hepatitis C virus replication from genome to function. Nature 2005; 436: 933–938.
Li T, Li D, Cheng L, Wu H, Gao Z, Liu Z et al. Epithelial-mesenchymal transition induced by hepatitis C virus core protein in cholangiocarcinoma. Ann Surg Oncol 2010; 17: 1937–1944.
Akkari L, Grégoire D, Floc'h N, Moreau M, Hernandez C, Simonin Y et al. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J Hepatol 2012; 57: 1021–1028.
Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012; 61: 439–448.
von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 2009; 137: 361–371.
Peinado H, Ballestar E, Esteller M, Cano A . Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 2004; 24: 306–319.
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 2012; 31: 583–594.
Ropero S, Esteller M . The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007; 1: 19–25.
Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ, Kim HS et al. HDAC2 provides a critical support to malignant progression of hepatocellular carcinoma through feedback control of mTORC1 and AKT. Cancer Res 2014; 74: 1728–1738.
Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN . The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain SNAG, inhibits G1 arrest induced by interleukin-2 withdrawal. Mol Cell Biol 1996; 16: 6263–6272.
Mingot JM, Vega S, Maestro B, Sanz JM, Nieto MA . Characterization of Snail nuclear import pathways as representatives of C2H2 zinc finger transcription factors. J Cell Sci 2009; 122: 1452–1460.
Hou Z, Peng H, White DE, Wang P, Lieberman PM, Halazonetis T et al. 14-3-3 binding sites in the snail protein are essential for snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res 2010; 70: 4385–4393.
Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ . Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem 2005; 280: 11740–11748.
MacPherson MR, Molina P, Souchelnytskyi S, Wernstedt C, Martin-Pérez J, Portillo F et al. Phosphorylation of serine 11 and serine 92 as new positive regulators of human Snail1 function: potential involvement of casein kinase-2 and the cAMP-activated kinase protein kinase A. Mol Biol Cell 2010; 21: 244–253.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6: 931–940.
Domínguez D, Montserrat-Sentís B, Virgós-Soler A, Guaita S, Grueso J, Porta M et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol 2003; 23: 5078–5089.
Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS . Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J Biol Chem 2011; 286: 10847–10855.
Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010; 143: 1136–1148.
Xie Q, Chen L, Shan X, Shan X, Tang J, Zhou F et al. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int J Cancer 2014; 135: 635–646.
Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC et al. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem 2009; 108: 295–303.
Yang D, Zuo C, Wang X, Meng X, Xue B, Liu N et al. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proc Natl Acad Sci USA 2014; 111: E1264–E1273.
Sideridou M, Zakopoulou R, Evangelou K, Liontos M, Kotsinas A, Rampakakis E et al. Cdc6 expression represses E-cadherin transcription and activates adjacent replication origins. J Cell Biol 2011; 195: 1123–1140.
Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 2009; 13: 2448–2464.
Su Y, Wagner ER, Luo Q, Huang J, Chen L, He BC et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene 2011; 30: 3907–3917.
Acknowledgements
We would like to thank Dr T-C He (University of Chicago, USA) for the kind provision of adenoviruses AdGFP, AdRFP, and AdR-FLuc. We are also grateful to Dr Takaji Wakita (National Institute of Infectious Diseases, Tokyo) for providing an HCV JFH1 cDNA clone and to Dr Haizhen Zhu (Hunan University, China) for the HLCZ01 cells. This study was supported by research grants from Major National S&T program (2013ZX10002002-005-003, NT; 2012ZX10002005-003-002, NT), China National Natural Science Foundation (#81572683, #81371827, #31171307, NT; #81502062, DN), Innovation Fund Designated for Graduate Students of Chongqing Government (#CYS14124, LN) and the Outstanding Young talent program of 2nd affiliated hospital of CQMU (NT).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Nie, D., Shan, X., Nie, L. et al. Hepatitis C virus core protein interacts with Snail and histone deacetylases to promote the metastasis of hepatocellular carcinoma. Oncogene 35, 3626–3635 (2016). https://doi.org/10.1038/onc.2015.428
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2015.428
This article is cited by
-
The role and application of transcriptional repressors in cancer treatment
Archives of Pharmacal Research (2023)
-
EVI1 promotes epithelial-to-mesenchymal transition, cancer stem cell features and chemo−/radioresistance in nasopharyngeal carcinoma
Journal of Experimental & Clinical Cancer Research (2019)
-
Regulation of miRNAs by Snail during epithelial-to-mesenchymal transition in HT29 colon cancer cells
Scientific Reports (2019)