Abstract
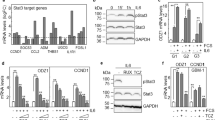
Long-term survival remains low for most patients with glioblastoma (GBM), which reveals the need for markers of disease outcome and novel therapeutic targets. We describe that ODZ1 (also known as TENM1), a type II transmembrane protein involved in fetal brain development, plays a crucial role in the invasion of GBM cells. Differentiation of glioblastoma stem-like cells drives the nuclear translocation of an intracellular fragment of ODZ1 through proteolytic cleavage by signal peptide peptidase-like 2a. The intracellular fragment of ODZ1 promotes cytoskeletal remodelling of GBM cells and invasion of the surrounding environment both in vitro and in vivo. Absence of ODZ1 by gene deletion or downregulation of ODZ1 by small interfering RNAs drastically reduces the invasive capacity of GBM cells. This activity is mediated by an ODZ1-triggered transcriptional pathway, through the E-box binding Myc protein, that promotes the expression and activation of Ras homolog family member A (RhoA) and subsequent activation of Rho-associated, coiled-coil containing protein kinase (ROCK). Overexpression of ODZ1 in GBM cells reduced survival of xenografted mice. Consistently, analysis of 122 GBM tumour samples revealed that the number of ODZ1-positive cells inversely correlated with overall and progression-free survival. Our findings establish a novel marker of invading GBM cells and consequently a potential marker of disease progression and a therapeutic target in GBM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 2010; 16: 2443–2449.
Giese A, Bjerkvig R, Berens ME, Westphal M . Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 2003; 21: 1624–1636.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006; 9: 157–173.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Yan K, Yang K, Rich JN . The evolving landscape of glioblastoma stem cells. Curr Opin Neurol 2013; 26: 701–707.
Roussel MF, Robinson GW . Role of MYC in medulloblastoma. Cold Spring Harb Perspect Med 2013; 3: a014308.
Kobayashi K, Hatano M, Otaki M, Ogasawara T, Tokuhisa T . Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc Nat Acad Sci USA 1999; 96: 1457–1462.
Ambrosini G, Adida C, Altieri DC . A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3: 917–921.
Teglund S, Toftgard R . Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010; 1805: 181–208.
Tucker RP, Beckmann J, Leachman NT, Scholer J, Chiquet-Ehrismann R . Phylogenetic analysis of the teneurins: conserved features and premetazoan ancestry. Mol Biol Evol 2012; 29: 1019–1029.
Kenzelmann D, Chiquet-Ehrismann R, Leachman NT, Tucker RP . Teneurin-1 is expressed in interconnected regions of the developing brain and is processed in vivo. BMC Dev Biol 2008; 8: 30.
Nunes SM, Ferralli J, Choi K, Brown-Luedi M, Minet AD, Chiquet-Ehrismann R . The intracellular domain of teneurin-1 interacts with MBD1 and CAP/ponsin resulting in subcellular codistribution and translocation to the nuclear matrix. Exp Cell Res 2005; 305: 122–132.
Schoeler J, Ferralli J, Thiry S, Chiquet-Ehrismann R . The intracellular domain of teneurin-1 induces the activity of transcription factor MITF by binding to transcriptional repressor HINT1. J Biol Chem 2015; 290: 8154–8165.
Zhang W, Zang Z, Song Y, Yang H, Yin Q . Co-expression network analysis of differentially expressed genes associated with metastasis in prolactin pituitary tumors. Mol Med Rep 2014; 10: 113–118.
Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Nat Acad Sci USA 2001; 98: 15044–15049.
Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L . Trans-synaptic teneurin signalling in neuromuscular synapse organization and target choice. Nature 2012; 484: 237–241.
Ruiz-Ontanon P, Orgaz JL, Aldaz B, Elosegui-Artola A, Martino J, Berciano MT et al. Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells 2013; 31: 1075–1085.
Jaffe AB, Hall A . Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21: 247–269.
Ernst A, Hofmann S, Ahmadi R, Becker N, Korshunov A, Engel F et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res 2009; 15: 6541–6550.
Jin X, Sohn YW, Yin J, Kim SH, Joshi K, Nam DH et al. Blockade of EGFR signaling promotes glioma stem-like cell invasiveness by abolishing ID3-mediated inhibition of p27(KIP1) and MMP3 expression. Cancer Lett 2013; 328: 235–242.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Mazzoleni S, Politi LS, Pala M, Cominelli M, Franzin A, Sergi Sergi L et al. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res 2010; 70: 7500–7513.
Nogueira L, Ruiz-Ontanon P, Vazquez-Barquero A, Lafarga M, Berciano MT, Aldaz B et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene 2011; 30: 3537–3548.
Zhang M, Liu J, Cheng A, Deyoung SM, Chen X, Dold LH et al. CAP interacts with cytoskeletal proteins and regulates adhesion-mediated ERK activation and motility. EMBO J 2006; 25: 5284–5293.
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012; 22: 21–35.
Heasman SJ, Ridley AJ . Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9: 690–701.
Sahai E, Marshall CJ . RHO-GTPases and cancer. Nat Rev Cancer 2002; 2: 133–142.
Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y et al. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res 2004; 10: 4799–4805.
Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol 2010; 12: 457–467.
Canelles M, Delgado MD, Hyland KM, Lerga A, Richard C, Dang CV et al. Max and inhibitory c-Myc mutants induce erythroid differentiation and resistance to apoptosis in human myeloid leukemia cells. Oncogene 1997; 14: 1315–1327.
Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y et al. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res 2009; 7: 570–580.
Lee K, Song K . Actin dysfunction activates ERK1/2 and delays entry into mitosis in mammalian cells. Cell Cycle 2007; 6: 1487–1495.
Ying Z, Li Y, Wu J, Zhu X, Yang Y, Tian H et al. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res 2013; 73: 990–999.
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol 2012; 189: 444–453.
Sherry MM, Reeves A, Wu JK, Cochran BH . STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells 2009; 27: 2383–2392.
Yan B, Chour HH, Peh BK, Lim C, Salto-Tellez M . RhoA protein expression correlates positively with degree of malignancy in astrocytomas. Neurosci Lett 2006; 407: 124–126.
Kirkin V, Cahuzac N, Guardiola-Serrano F, Huault S, Luckerath K, Friedmann E et al. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ 2007; 14: 1678–1687.
Shervington A, Cruickshanks N, Wright H, Atkinson-Dell R, Lea R, Roberts G et al. Glioma: what is the role of c-Myc, hsp90 and telomerase? Mol Cell Biochem 2006; 283: 1–9.
Shindo H, Tani E, Matsumuto T, Hashimoto T, Furuyama J . Stabilization of c-myc protein in human glioma cells. Acta Neuropathol 1993; 86: 345–352.
Timpson P, McGhee EJ, Morton JP, von Kriegsheim A, Schwarz JP, Karim SA et al. Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res 2011; 71: 747–757.
Jacquemet G, Green DM, Bridgewater RE, von Kriegsheim A, Humphries MJ, Norman JC et al. RCP-driven alpha5beta1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J Cell Biol 2013; 202: 917–935.
Deng L, Li G, Li R, Liu Q, He Q, Zhang J . Rho-kinase inhibitor, fasudil, suppresses glioblastoma cell line progression in vitro and in vivo. Cancer Biol Ther 2010; 9: 875–884.
Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M . Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res 2009; 29: 119–123.
Hirata E, Yukinaga H, Kamioka Y, Arakawa Y, Miyamoto S, Okada T et al. in vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J Cell Sci 2012; 125: 858–868.
Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin JM, Martuscello RT et al. Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res 2015; 75: 1113–1122.
Wang H, Han M, Whetsell W Jr, Wang J, Rich J, Hallahan D et al. Tax-interacting protein 1 coordinates the spatiotemporal activation of Rho GTPases and regulates the infiltrative growth of human glioblastoma. Oncogene 2014; 33: 1558–1569.
Danussi C, Akavia UD, Niola F, Jovic A, Lasorella A, Pe'er D et al. RHPN2 drives mesenchymal transformation in malignant glioma by triggering RhoA activation. Cancer Res 2013; 73: 5140–5150.
Tost J, Gut IG . DNA methylation analysis by pyrosequencing. Nat Protoc 2007; 2: 2265–2275.
Pozo N, Zahonero C, Fernandez P, Linares JM, Ayuso A, Hagiwara M et al. Inhibition of DYRK1A destabilizes EGFR and reduces EGFR-dependent glioblastoma growth. J Clin Invest 2013; 123: 2475–2487.
Watsuji T, Okamoto Y, Emi N, Katsuoka Y, Hagiwara M . Controlled gene expression with a reverse tetracycline-regulated retroviral vector (RTRV) system. Biochem Biophys Res Commun 1997; 234: 769–773.
Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH . Myc confers androgen-independent prostate cancer cell growth. J Clin Invest 2003; 112: 1724–1731.
Grande L, Bretones G, Rosa-Garrido M, Garrido-Martin EM, Hernandez T, Fraile S et al. Transcription factors Sp1 and p73 control the expression of the proapoptotic protein NOXA in the response of testicular embryonal carcinoma cells to cisplatin. J Biol Chem 2012; 287: 26495–26505.
Acknowledgements
This work was supported by the Instituto de Salud Carlos III (ISCIII), grants PI13/01760 (JLF-L), PI12/00775 (PS-G) and SAF2014-53526R (JL), and program Red Temática de Investigación Cooperativa en Cáncer (RTICC) grants RD12/0036/0022 (JLF-L), RD12/0036/0027 (PS-G), RD12/0036/0063 (JAM-C), RD12/0036/0003 (AP) and RD12-0036-0033 (JL), Instituto de Investigación Valdecilla (IDIVAL) grant APG/03 to JLF-L, and Cancer Research UK C33043/A12065 and Royal Society RG110591 to VS-M. We thank R. Galli for kindly providing RG1 cell line. We also thank M.E. Fernández-Valle for helping with magnetic resonance imaging experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Talamillo, A., Grande, L., Ruiz-Ontañon, P. et al. ODZ1 allows glioblastoma to sustain invasiveness through a Myc-dependent transcriptional upregulation of RhoA. Oncogene 36, 1733–1744 (2017). https://doi.org/10.1038/onc.2016.341
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2016.341
This article is cited by
-
RETRACTED ARTICLE: Downregulation of the Rho GTPase pathway abrogates resistance to ionizing radiation in wild-type p53 glioblastoma by suppressing DNA repair mechanisms
Cell Death & Disease (2023)
-
Molecular signature of excessive female aggression: study of stressed mice with genetic inactivation of neuronal serotonin synthesis
Journal of Neural Transmission (2023)
-
Glioblastoma invasion factor ODZ1 is induced by microenvironmental signals through activation of a Stat3-dependent transcriptional pathway
Scientific Reports (2021)
-
MEX3A contributes to development and progression of glioma through regulating cell proliferation and cell migration and targeting CCL2
Cell Death & Disease (2021)
-
Alternative splicing controls teneurin-latrophilin interaction and synapse specificity by a shape-shifting mechanism
Nature Communications (2020)