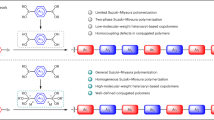
Abstract
We report the synthesis and properties of π-stacked polymers consisting of oligothiophene and naphthalene as the stacked π-system and the scaffold, respectively. The titled polymers were obtained by the Suzuki–Miyaura coupling reaction. Oligothiophene units were layered in proximity, ∼3.0 Å from each other. Contribution of the quinoidal structure of the oligothiophene units involving the naphthalene scaffolds in the excited state resulted in relatively high photoluminescence quantum efficiencies. The polymers have potential application to optoelectronic devices such as hole-transporting materials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Salaneck, W. R., Clark, D. T. & Samuelsen, E. J. (eds). Science and Applications of Conducting Polymers, (Adam Hilger: Bristol, 1991).
Nalwa, H. S. (ed.) Handbook of Organic Conductive Molecules, (Wiley: Chichester, 1997).
Skotheim, T. A., Elsenbaumer, R. L. & Reynolds, J. R. (eds). Hand book of Conducting Polymers, 3rd edn(Marcel Dekker: New York, 2006).
Nakano, T. Synthesis, structure and function of π-stacked polymers. Polym. J. 42, 103–123 (2010).
Nakano, T., Takewaki, K., Yade, T. & Okamoto, Y. Dibenzofulvene, a 1,1-diphenylethylene analogue, gives a π-stacked polymer by anionic, free-radical, and cationic catalysts. J. Am. Chem. Soc. 123, 9182–9183 (2001).
Nakano, T. & Yade, T. Synthesis, structure, and photophysical and electrochemical properties of a π-stacked polymer. J. Am. Chem. Soc. 125, 15474–15484 (2003).
Nakano, T., Yade, T., Yokoyama, M. & Nagayama, N. Charge transport in a π-stacked poly(divenzofulvene) film. Chem. Lett. 33, 296–297 (2004).
Nakano, T., Yade, T., Fukuda, Y., Yamaguchi, T. & Okumura, S. Free-radical polymerization of divenzofulvene leading to a π-stacked polymer: structure and properties of the polymer and proposed reaction mechanism. Macromolecules 38, 8140–8148 (2005).
Yade, T. & Nakano, T. Anionic polymerization of 2,7-di-t-butyldivenzofulvene synthesis, structure, and photophysical properties of the oligomers with a π-stacked conformation. J. Polym. Sci.: Part A: Polym. Chem. 44, 561–572 (2006).
Nakano, T. & Yade, T. Charge delocalization over stacked π-electron systems. Chem. Lett. 37, 258–259 (2008).
Nakano, T., Tanikawa, M., Nakagawa, O., Yade, T. & Sakamoto, T. Synthesis and structure of an optically active π-stacked poly(dibenzofulvene) bearing chiral terminal group. J. Polym. Sci.: Part A: Polym. Chem. 47, 239–246 (2009).
García Martínez, A., Osío Barcina, J., de Fresno Cerezo, A., Schlüter, A.- D. & Frahn, J. Synthesis of poly[p-(7-phenylene-7-(2′,5′-dihexyl-4-biphenylene))norbornane]: the first soluble polymer with alternating conjugation and homoconjugation. Adv. Mater. 11, 27–31 (1999).
Osío Barcina, J., Colorado Heras, M. R., Mba, M., Gómez Aspe, R. & Herrero-García, N. Efficient electron delocalization mediated by aromatic homoconjugation in 7,7-diphenylnorbornane derivatives. J. Org. Chem. 74, 7148–7156 (2009).
Morisaki, Y. & Chujo, Y. Through-space conjugated polymers based on cyclophanes. Angew. Chem., Int. Ed. 45, 6430–6437 (2006).
Morisaki, Y. & Chujo, Y. Cyclophane-containing polymers. Prog. Polym. Sci. 33, 346–364 (2008).
Morisaki, Y. & Chujo, Y. Synthesis of π-stacked polymers on the basis of paracyclophane. Bull. Chem. Soc. Jpn. 82, 1070–1082 (2009).
Morisaki, Y. & Chujo, Y. Synthesis of novel π-conjugated polymers having paracyclophane skeleton in the main chain. Extension of π-conjugated length via the through-space. Macromolecules 35, 587–589 (2002).
Morisaki, Y. & Chujo, Y. Synthesis of novel alternating π-conjugated copolymers having paracyclophane and fluorene units in the main chain leading to the blue light-emitting materials. Chem. Lett. 194–195 (2002).
Morisaki, Y., Ishida, T. & Chujo, Y. Synthesis and properties of novel through-space π-conjugated polymers based on poly(p-phenylenevinylene)s having a paracyclophane skeleton in the main chain. Macromolecules 35, 7872–7877 (2002).
Morisaki, Y., Fujimura, F. & Chujo, Y. Synthesis and properties of novel σ-π conjugated polymers with alternating organosilicon and paracyclophane units in the main chain. Organometallics 22, 3553–3557 (2003).
Morisaki, Y. & Chujo, Y. Synthesis and properties of a novel through-space conjugated polymer with paracyclophane and ferrocene in the main chain. Macromolecules 36, 9319–9324 (2003).
Morisaki, Y., Ishida, T., Tanaka, H. & Chujo, Y. Synthesis and properties of the paracyclophane-containing conjugated polymer with benzothiadiazole as an electron accepter. J. Polym. Sci.: Part A: Polym. Chem. 42, 5891–5899 (2004).
Morisaki, Y. & Chujo, Y. Novel paracyclophane-fluorene-based conjugated copolymers: synthesis, optical, and electrochemical properties. Macromolecules 37, 4099–4103 (2004).
Morisaki, Y. & Chujo, Y. Novel through-space conjugated polymers consisting of alternate paracyclophane and fluorene. Bull. Chem. Soc. Jpn. 78, 288–293 (2005).
Morisaki, Y., Shiotani, Y., Lin, L. & Chujo, Y. Synthesis of PAMAM dendrimers possessing paracyclophane on their surface. Polym. J. 40, 779–783 (2008).
Wada, N., Morisaki, Y. & Chujo, Y. Polymethylenes containing paracyclophane in the side chain. Macromolecules 42, 1439–1442 (2009).
Morisaki, Y., Lin, L. & Chujo, Y. Synthesis of cyano-substituted through-space poly(p-arylenevinylene). Chem. Lett. 38, 734–735 (2009).
Morisaki, Y., Lin, L. & Chujo, Y. Synthesis and properties of through-space conjugated polymers based on cyano-substituted poly(p-arylenevinylene)s. J. Polym. Sci.: Part A: Polym. Chem. 47, 5979–5988 (2009).
Lin, L., Morisaki, Y. & Chujo, Y. Synthesis of through-space conjugated polymers containing paracyclophane and thienopyrazine in the main chain. J. Polym. Sci.: Part A: Polym. Chem. 47, 7003–7011 (2009).
Guyard, L. & Audebert, P. Synthesis and electrochemical polymerization of bis-dithienyl cyclophane. Electrochem. Commun. 3, 164–167 (2001).
Guyard, L., Audebert, P., Dolbier, W. R. Jr. & Duan, J.- X. Synthesis and electrochemical polymerization of new oligothiophene functionalized fluorocyclophane. J. Electroanal. Chem. 537, 189–193 (2002).
Salhi, F., Lee, B., Metz, C. & Bottomley, L. A. Influenece of π-stacking on the redox properties of oligothiophenes: (α-alkyloligo-thienyl)paracyclophane. Org. Lett. 4, 3195–3198 (2002).
Salhi, F. & Collard, D. M. π-Stacked conjugated polymers: the influence of paracyclophane π-stackes on the redox and optical properties of a new class of broken conjugated polythiophenes. Adv. Mater. 15, 81–85 (2003).
Mizogami, S. & Yoshimura, S. Synthesis of a new polycojugated system: polycyclophane. J. Chem. Soc., Chem. Commun. 427–428 (1985).
Mizogami, S. & Yoshimura, S. Synthesis of a new crystalline polymer: polymetacyclophane. J. Chem. Soc., Chem. Commun. 1736–1737 (1985).
Nugent, H. M., Rosenblum, M. & Klemarczky, P. Synthesis of face-to-face metallocene polymers. J. Am. Chem. Soc. 115, 3848–3849 (1993).
Rosenblum, M., Nugent, H. M., Jang, K.- S., Labes, M. M., Cahalane, W., Klemarczyk, P. & Reiff, W. M. The synthesis and properties of face-to-face metallocene polymers. Macromolecules 28, 6330–6342 (1995).
Hudson, R. D. A., Foxman, B. M. & Rosenblum, M. Synthesis and properties of new stacked metallocene polymers. Organometallics 18, 4098–4106 (1999).
Jenekhe, S. A., Alam, M. M., Zhu, Y. & Jiang, S. Single-molecule nanomaterials from π-stacked side-chain conjugated polymers. Adv. Mater. 19, 536–542 (2007).
Morisaki, Y. & Chujo, Y. Construction of benzene ring-layered polymers. Tetrahedron Lett. 46, 2533–2537 (2005).
Morisaki, Y., Murakami, T. & Chujo, Y. Synthesis and properties of paracyclophane-layered polymers. Macromolecules 41, 5960–5963 (2008).
Morisaki, Y., Murakami, T. & Chujo, Y. Synthesis, structure, and properties of aromatic ring-layered polymers containing ferrocene as a terminal unit. J. Inorg. Organomet. Polym. Mater. 19, 104–112 (2009).
Morisaki, Y., Murakami, T., Sawamura, T. & Chujo, Y. Paracyclophane-layered polymers end-capped with fluorescence quenchers. Macromolecules 42, 3656–3660 (2009).
Morisaki, Y., Imoto, H., Miyake, J. & Chujo, Y. Synthesis and properties of oligophenylene-layered polymers. Macromol. Rapid Commun. 30, 1094–1100 (2009).
Morisaki, Y., Fernandes, J. A., Wada, N. & Chujo, Y. Synthesis and properties of carbazole-layered polymers. J. Polym. Sci.: Part A: Polym. Chem. 47, 4279–4288 (2009).
Morisaki, Y., Fernandes, J. A. & Chujo, Y. Synthesis of oligothiophene-layered polymers. Macromol. Rapid Commun. 30, 2107–2111 (2009).
Morisaki, Y., Sawamura, T., Murakami, T. & Chujo, Y. Synthesis of anthracene-stacked oligomers and polymer. Org. Lett. 12, 3188–3191 (2010).
Oligothiophene-layered polymers based on o-substituted benzene have been reported Tovar, J. D. & Swager, T. M. Cofacially constrained organic semiconductors. J. Polym. Sci.: Part A: Polym. Chem. 41, 3693–3702 (2003).
Oligothiophene-layered polymers based on calyxarenas Yu, H.- H. Xu, B. & Swager, T. M. A proton-doped calixarene-based conducting polymer. J. Am. Chem. Soc. 125, 1142–1143 (2003), See also reference 50..
Yu, H.-h., Pullen, A. E., Büschel, M. G. & Swager, T.M. Conducting Polymer Actuator Mechanism Based on the Conformational Flexibility of Calix[4]arene. Angew. Chem., Int. Ed. 43, 3700–3703 (2004).
Vinyl polymers containing oligothiophenes in the side chain were synthesized; see Melucci, M. Barbarella, G. Zambianchi, M. Benzi, M. Biscarini, F. Cavallini, M. Bongini, A. Fabbroni, S. Mazzeo, M. Anni, M. & Gigli, G. Poly(α-vinyl-ω-alkyloligothiophene) side-chain polymers. synthesis, fluorescence, and morphology. Macromolecules 37, 5692–5702 (2004).
Sangvikar, Y., Fischer, K., Schmidt, M., Schlüter, A. D. & Sakamoto, J. Suzuki polycondensation with a hairpin monomer. Org. Lett. 11, 4112–4115 (2009).
Chou, C.- M., Lee, S.- L., Chen, C.- H., Biju, A. T., Wang, H. W., Wu, Y. L., Zhang, G.- F., Yang, K.- W., Lim, T- S., Huang, M- J., Tsai, P.- Y., Lin, K.- C., Huang, S.- L., Chen, C.-h. & Luh, T.- Y. Polymeric Ladderphanes. J. Am. Chem. Soc. 131, 12579–12585 (2009).
Schuster, G. B. (ed.) Long-range Charge Transfer in DNA I & II (Springer: Berlin, 2004).
Sundar, V. C., Zaumseil, J., Podzorov, V., Menard, E., Willett, R. L., Someya, T., Gershenson, M. E. & Rogers, J. A. Elastomeric transistor stamps: reversible probing of charge transport in organic crystals. Science 303, 1644–1646 (2004).
Otsubo, T., Mizogami, S., Otsubo, I., Tozuka, Z., Sakagami, A., Sakata, Y. & Misumi, S. Layered compounds. XV. synthesis and properties of multilayered cyclophanes. Bull. Chem. Soc. Jpn. 46, 3519–3530 (1973).
Misumi, S. & Otsubo, T. Chemistry of multilayered cyclophanes. Acc. Chem. Res. 11, 251–256 (1978).
Shibahara, M., Watanabe, M., Iwanaga, T. & Shinmyozu, T. Synthesis, structure, and transannular π-π interaction of multilayered metacyclophanes. J. Org. Chem. 72, 2865–2877 (2007).
Shibahara, M., Watababe, M., Iwanaga, T., Matsumoto, T., Ideta, K. & Shinmyozu, T. Synthesis, structure, and transannular π-π interaction of three- and four-layered paracyclophanes. J. Org. Chem. 73, 4433–4442 (2008).
Kuroda, M., Nakayama, J. & Hoshino, M. Synthesis and properties of naphthalenes carrying two cofacially oriented α-oligothiophene units at the peri positions. Tetrahedron Lett. 33, 7553–7556 (1992).
Kuroda, M., Nakayama, J. & Hoshino, M. Synthesis and properties of 1,8-di(2-thienyl)-, 1,8-bis(5,2′-bithiophene-2-yl)-, 1,8-bis(5,2′:5′,2′′-terthiophene-2-yl)-, and 1,8-bis(5,2′:5′,2′′:5′′,2′′′-quaterthiophene-2-yl)naphthalenes and related compounds. Tetrahedron 49, 3735–3748 (1993).
Kuroda, M., Nakayama, J., Hoshino, M., Furusho, N. & Ohba, S. Synthesis and properties of α-oligothiophenes carrying three cofacially oriented thiophene rings through peri positions of naphthalenes. Tetrahedron Lett. 35, 3957–3960 (1994).
Iyoda, M., Nakao, K., Kondo, T., Kuwatani, Y., Yoshida, M., Matsuyama, H., Fukami, K. & Nagase, S. (1,8)Naphthalenophane containing 2,2′-bithienyl-5,5′-ylene bridges. Tetrahedron Lett. 42, 6869–6872 (2001).
Nakao, K., Nishiguchi, T. & Iyoda, M. Syntheses, structures, and properties of bithiophenophanes bridged at 1,8-positions of naphthalenes. Heterocycles 76, 727–745 (2008).
Pangborn, A. B., Giardello, M. A., Grubbs, R. H., Rosen, R. K. & Timmers, F. J. Safe and convenient procedure for solvent purification. Organometallics 15, 1518–1520 (1996).
House, H. O., Koepsell, D. G. & Campbell, W. J. Synthesis of some diphenyl and triphenyl derivatives of anthracene and naphthalene. J. Org. Chem. 37, 1003–1011 (1972).
Tang, W., Singh, S. P., Ong, K. H. & Chen, Z.- K. Synthesis of thienothiophene derived conjugated oligomers for field-effect transistors applications. J. Mater. Chem. 20, 1497–1505 (2010).
Miyaura, N. & Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc., Chem. Commun. 866–867 (1979).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Walker, S. D., Barder, T. E., Martinelli, J. R. & Buchwald, S L A Rationally designed universal catalyst for Suzuki-Miyaura coupling processes. Angew. Chem., Int. Ed. 43, 1871–1876 (2004).
Negishi, E. In: Handbook of Organopalladium Chemistry for Organic Synthesis, (ed. Negishi, E.) 229–247, (Wiley-VCH: New York, 2002).
Arbizzani, C., Bongini, A., Mastragostino, M., Zanelli, A., Barbarella, G. & Zambianchi, M. Polyalkylthiophenes as electrochromic materials: a comparative study of poly(3-methylthiophenes) and poly(3-hexylthiophenes). Adv. Mater. 7, 571–574 (1995).
Synthesis and X-ray crystal structures of 3,4-di(2-thienyl)-7,7-7H-2,5-dithia-7-silacyclopenta[a]pentalene was reported, in which the shortest distance between two thiophene rings was 3.44 Å; see Iyoda, M. Copper-mediated aryl-aryl couplings for the construction of oligophenylenes and related heteroaromatics. Adv. Synth. Catal. 351, 984–998 (2009).
Xu, B. & Holdcroft, S. Molecular control of luminescence from poly(3-hexylthiophenes). Macromolecules 26, 4457–4460 (1993).
Seixas de Melo, J., Silva, L. M. & Kuroda, M. Photophysical and theoretical studies of naphthalene-substituted oligothiophenes. J. Chem. Phys. 115, 5625–5636 (2001).
Pina, J. & Seixas de Melo, J. A comprehensive investigation of the electronic spectral and photophysical properties of conjugated naphthalene-thiophene oligomers. Phys. Chem. Chem. Phys. 11, 8706–8713 (2009).
Chosrovian, H., Rentsch, S., Grebner, D., Dahm, D. U. & Birckner, E. Time-resolved fluorescence studies on thiophene oligomers in solution. Synth. Met. 60, 23–26 (1993).
These values were calculated from the Eonset of the polymers and the energy level of Fc/Fc+ (4.80 eV), namely HOMO= −4.80—Eonset. For example, see Pommerehne, J., Vestweber, H., Guss, W., Mahrt, R. F., Bässler, H., Porsch, M., & Daub, J. Efficient two layer LEDs on a polymer blend basis. Adv. Mater. 7, 551–554 (1995).
Acknowledgements
This work was supported by Grant-in-Aid for Young Scientists (A) (No. 21685012) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Morisaki, Y., Fernandes, J. & Chujo, Y. Naphthalene-based oligothiophene-stacked polymers. Polym J 42, 928–934 (2010). https://doi.org/10.1038/pj.2010.101
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2010.101
Keywords
This article is cited by
-
π-Conjugated polymer-layered structures: synthesis and self-assembly
Polymer Journal (2017)