Abstract
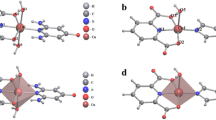
Imidazole-terminated first- and second-generation poly(amidoamine)-type oligomeric silsesquioxanes (POSS)-core dendrimers (denoted as POSS-Im16 and POSS-Im32, respectively) were synthesized by the ester–amide exchange reaction from first- and second-generation methyl ester-terminated POSS-core dendrimers, respectively. Transmittances of the aqueous POSS-Im32 solution drastically decreased at pH>7.2, but the change was reversible without hysteresis. The results of pH titrations for POSS-Im16 and POSS-Im32 showed well-defined one-step titration curves identical to those of monomeric imidazole compounds, such as 1-methylimidazole. Such simple acid–base behavior caused phase transition in the aqueous dendrimer solution to be highly sensitive to pH changes. The pKa values of 1-methylimidazole, POSS-Im16 and POSS-Im32 were 7.3, 7.0 and 6.7, respectively. Spectrophotometric titrations of the aqueous dendrimer solutions with Cu2+ ions indicated that coordination modes of POSS-Im16 changed from the Cu2+–N2O2 complex to the Cu2+–N4 complex as the concentration of the dendrimers increased; however, only one complexation mode (Cu2+–N4) existed between Cu2+ ions and POSS-Im32.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Feher, F. J. & Wyndham, K. D. Amine and ester-substituted silsesquioxanes: synthesis, characterization and use as a core for starburst dendrimers. Chem. Commun. 34, 323–324 (1998).
Feher, F. J., Wyndham, K. D., Soulivong, D. & Nguyen, F. Syntheses of highly functionalized cube-octameric polyhedral oligosilsesquioxanes (R8Si8O12). J. Chem. Soc. Dalton Trans. 28, 1491–1497 (1999).
Jaffrés, P.- A. & Morris, R. E. Synthesis of highly functionalized dendrimers based on polyhedral silsesquioxane cores. J. Chem. Soc. Dalton Trans. 27, 2767–2770 (1998).
Zhang, X., Haxton, K. J., Ropartz, L., Cole-Hamilton, D. J. & Morris, R. E. Synthesis and computer modeling of hydroxy-derivatised carbosilane dendrimers based on polyhedral silsesquioxane cores. J. Chem. Soc. Dalton Trans. 30, 3261–3268 (2001).
Haxton, K. J., Cole-Hamilton, D. J. & Morris, R. E. Silsesquioxane dendrimers as catalysts: a bite-sized molecular dynamics study. Dalton Trans. 36, 3415–3420 (2007).
Naka, K., Fujita, M., Tanaka, K. & Chujo, Y. Water-soluble anionic POSS-core dendrimer: synthesis and copper (II) complexes in aqueous solution. Langmuir 23, 9057–9063 (2007).
Tanaka, K., Inafuku, K., Naka, K. & Chujo, Y. Enhancement of entrapping ability of dendrimers by a cubic silsesquioxane core. Org. Biomol. Chem. 6, 3899–3901 (2008).
Haba, Y., Harada, A., Takagishi, T. & Kono, K. Rendering poly(amidoamine) or poly(propylenimine) dendrimers temperature sensitive. J. Am. Chem. Soc. 126, 12760–12761 (2004).
Haba, Y., Kojima, C., Harada, A. & Kono, K. Control of temperature-sensitive properties of poly(amidoamine) dendrimers using peripheral modification with various alkylamide groups. Macromolecules 39, 7451–7453 (2006).
You, Y. Z., Hong, C. Y., Pan, C. Y. & Wang, P. H. Synthesis of a dendritic core-shell nanostructure with a temperature-sensitive shell. Adv. Mater. 16, 1953–1957 (2004).
Li, W., Zhang, A., Chen, Y., Feldman, K., Wu, H. & Schlüter, A. D. Low toxic, thermoresponsive dendrimers based on oligoethylene glycols with sharp and fully reversible phase transitions. Chem. Commun. 44, 5948–5950 (2008).
Soler-Padrós, J., Pérez-Mayoral, E., Domínguez, L., López-Larrubia, P., Soriano, E., Marco-Contelles, J. L., Cerdán, S. & Ballesteros, P. Novel generation of pH indicators for proton magnetic resonance spectroscopic imaging. J. Med. Chem. 50, 4539–4542 (2007).
Seo, K. & Kim, D. Phase transition behavior of novel pH-sensitive polyaspartamide derivatives grafted with 1-(3-aminopropyl)imidazole. Macromol. Biosci. 6, 758–766 (2006).
Park, H. W., Jin, H.- S., Yang, S. Y. & Kim, J.- D. Tunable phase transition behaviors of pH-sensitive polyaspartamides having various cationic pendant groups. Colloid Polym. Sci. 287, 919–926 (2009).
Naka, K., Kobayashi, A. & Chujo, Y. Effect of anionic 4.5-generation polyamidoamine dendrimer on the formation of calcium carbonate polymorphs. Bull. Chem. Soc. Jpn 75, 2541–2546 (2002).
Baker, L. A., Sun, L. & Crooks, R. M. Synthesis and catalytic properties of imidazole-functionalized poly(propylene imine) dendrimers. Bull. Korean Chem. Soc. 23, 647–654 (2002).
Ojima, H. & Sone, K. Absorption spectra and catalytic behavior of the copper(II) chelates of some alkylated ethylenediamines. Bull. Chem. Soc. Jpn 35, 298–303 (1962).
Jonassen, H. B. & Dexter, T. H. Inorganic complex compounds containing polydentate groups. I. The complex ions formed between copper(II) ions and ethylenediamine. J. Am. Chem. Soc. 71, 1553–1556 (1949).
Edsall, J. D., Felsenfeld, G., Goodman, D. S. & Gurd, F. R. N. The association of imidazole with the ions of zinc and cupric copper. J. Am. Chem. Soc. 76, 3054–3061 (1953).
Jonassen, H. B., Reeves, R. E. & Segal, L. Inorganic complex compounds containing polydentate groups. XI. Effect of hydroxide ion on the bis-ethylenediaminecopper(II) ion. J. Am. Chem. Soc. 77, 2748–2749 (1955).
Billo, E. J. Copper(II) chromosomes and the rule of average environment. Inorg. Nucl. Chem. Lett. 10, 613–617 (1974).
Krot, K. A., Namor, A. F. D., Aguilar-Cornejo, A. & Nolan, K. B. Speciation, stability constants and structures of complexes of copper(II), nickel(II), silver(I) and mercury(II) with PAMAM dendrimer and related tetraamide ligands. Inorg. Chim. Acta 358, 3497–3505 (2005).
Acknowledgements
This study is a part of the Kyoto City Collaboration of Regional Entities for the Advancement of Technology Excellence of JST. We thank Professor Tsuyoshi Kawai of the Nara Institute of Science and Technology for making MALDI-TOF-MS measurements, which are supported by the Kyoto-Advance Nanotechnology Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Naka, K., Masuoka, S., Shinke, R. et al. Synthesis of first- and second-generation imidazole-terminated POSS-core dendrimers and their pH responsive and coordination properties. Polym J 44, 353–359 (2012). https://doi.org/10.1038/pj.2011.145
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2011.145
Keywords
This article is cited by
-
Thermo- and pH-sensitive Polymer with Pendant Spacer-linked Imidazole Cycles
Chinese Journal of Polymer Science (2023)
-
POSS cage-scrambling-induced gelation of POSS-pendant random copolymers catalyzed by fluoride anions
Polymer Journal (2021)
-
POSS ionic liquid crystals
NPG Asia Materials (2015)
-
Synthesis of imidazolium salt-terminated poly(amidoamine)-typed POSS-core dendrimers and their solution and bulk properties
Polymer Journal (2014)