Abstract
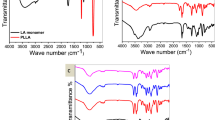
Mono-anthracene-terminated poly-L-lactide (A-PLLA) and mono-maleimide-terminated poly-D-lactide (M-PDLA) were prepared by ordinary lactide polymerization with initiators containing the corresponding functional groups. The resulting A-PLLA was allowed to dimerize by the reaction of its terminal hydroxyl with hexamethylene diisocyanate (HMDI) to obtain bis-anthracene-terminated PLLA (A-PLLA-A). The terminal Diels-Alder coupling between A-PLLA and M-PDLA and between A-PLLA-A and M-PDLA spontaneously gave stereo di- (d-sb-PLA) and tri-block (t-sb-PLA) copolymers, respectively, whose block sequences could be readily controlled by changing the molecular weight of the A-PLLA and M-PDLA prepolymers. The resultant d-sb-PLA and t-sb-PLA samples were found to have excellent thermal and thermo-mechanical properties due to the easy formation of stereocomplex crystals of PLLA and PDLA. These synthetic methods, which were based on polymer coupling, can be a facile route to the fabrication of stereo block polylactides possessing excellent properties.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kimura, Y. Molecular, structural, and material design of bio-based polymers design of bio-based polymers. Polymer. J. 41, 797–807 (2009).
Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 59, 145–152 (1998).
Ajioka, M., Enomoto, K., Suzuki, K. & Yamaguchi, A. The basic properties of poly(lactic acid) produced by the direct condensation polymerization of lactic acid. J. Environ. Polym. Degrad. 3, 225–234 (1995).
Moon, S. -I., Lee, C. -W., Taniguchi, I., Miyamoto, M. & Kimura, Y. Melt/solid polycondensation of L-lactic acid: an alternative route to poly(L-lactic acid) with high molecular weight. Polymer 42, 5059 (2001).
Dorgan, J. R., Lehermeier, H. & Mang, M. Thermal and Rheological Properties of commercial-grade poly(lactic acid)s. J. polym. Environ. 8, 1–9 (2000).
Ikada, Y., Jamshidi, K., Tsuji, H. & Hyon, S. H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 20, 904–906 (1987).
Tsuji, H., Hyon, S. H. & Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 3. Calorimetric studies on blend films cast from dilute solution. Macromolecules 24, 5651–5656 (1991).
Tsuji, H., Hyon, S. H. & Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 5. Calorimetric and morphological studies on the stereocomplex formed in acetonitrile solution. Macromolecules 25, 2940–2946 (1992).
Tsuji, H. & Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 6. Binary blends from copolymers. Macromolecules 25, 5719–5723 (1992).
Tsuji, H. & Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 26, 6918–6926 (1993).
Fukushima, K., Furuhashi, Y., Sogo, K., Miura, S. & Kimura, Y. Stereoblock poly(lactic acid): synthesis via solid-state polycondensation of a stereocomplexed mixture of Poly(L-lactic acid) and poly(D-lactic acid). Macromol. Biosci. 5, 21–29 (2005).
Fukushima, K. & Kimura, Y. A Novel synthetic approach to stereo-block poly(lactic acid). Macromol. Symp. 224, 133–144 (2005).
Fukushima, K. & Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym. Int. 55, 626–642 (2006).
Fukushima, K. & Kimura, Y. An Efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci., Part A: Polym. Chem. 46, 3714–3722 (2008).
Hirata, M. & Kimura, Y. Thermomechanical properties of stereoblock poly(lactic acid)s with different PLLA/PDLA block compositions. Polymer 49, 2656 (2008).
Kakuta, M., Hirata, M. & Kimura, Y. Stereoblock polylactides as high-performance bio-based polymers. J. Macromol. Sci., Part C: Polym. Rev. 49, 107–140 (2009).
Hirata, M., Kobayashi, K. & Kimura, Y. Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent D/L ratios. J. Polym. Sci., Part A: Polym. Chem. 48, 794–801 (2010).
Hirata, M., Kobayashi, K. & Kimura, Y. Enhanced stereocomplexation by enantiomer adjustment for stereo diblock polylactides with non-equivalent D/L Ratios. Macromol. Chem. Phys. 211, 1426–1431 (2010).
Masutani, K., Kawabata, S., Aoki, T. & Kimura, Y. Efficient formation of stereocomplexes of poly(L-lactide) and poly(D-lactide) by terminal Diels–Alder coupling. Polym. Int. 59, 1526–1530 (2010).
Jones, J. F., Liotta, C. L., Collard, D. M. & Schiraldi, D. A. Cross-linking and modification of poly(ethylene terephthalate-co-2,6-anthracenedicarboxylate) by diels−alder reactions with maleimides. Macromlecles 32, 5786–5792 (1999).
Brizzolara, D., Cantow, H. J., Diederichs, K., Keller, E. & Domb, A. J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 29, 191–197 (1996).
Acknowledgements
This work was part of a commissioned project, ‘Development of the Fundamental Technology on Green and Sustainable Chemical Processes’ that was supported by New Energy and Industrial Technology Development Organization (NEDO). Musashino Chemical Laboratory, Ltd. and Unitica Co., Ltd. are highly acknowledged for their support and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Masutani, K., Lee, C. & Kimura, Y. Synthesis and properties of stereo di- and tri-block polylactides of different block compositions by terminal Diels-Alder coupling of poly-L-lactide and poly-D-lactide prepolymers. Polym J 45, 427–435 (2013). https://doi.org/10.1038/pj.2012.161
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2012.161
Keywords
This article is cited by
-
Screening of crystalline species and enhanced nucleation of enantiomeric poly(lactide) systems by melt-quenching
Polymer Bulletin (2019)
-
Properties of stereo multi-block polylactides obtained by chain-extension of stereo tri-block polylactides consisting of poly(L-lactide) and poly(D-lactide)
Journal of Polymer Research (2018)
-
Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers
Scientific Reports (2017)
-
Stereocomplex crystallization and homo-crystallization of star-shaped four-armed stereo diblock poly(lactide)s during precipitation and non-isothermal crystallization
Polymer Journal (2016)