Abstract
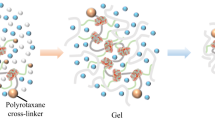
Stimuli-degradable cross-linked polymers were developed by applying both topological linkage and size complementarity of rotaxane to cross-link their structures. A double vinyl group-tethering [3]rotaxane cross-linker was prepared by reacting the axle ends of sec-ammonium/crown ether-type pseudo[3]rotaxane with a suitably bulky end-cap agent that was as large as the cavity of the wheel. Radical polymerization of a common vinyl monomer in the presence of the [3]rotaxane cross-linker afforded the corresponding stable cross-linked polymer under ambient conditions. An anion exchange reaction with tetra(n-butyl)ammonium chloride caused selective and efficient de-cross-linking of the cross-linked polymers to the vinyl polymer and the axle component of the cross-linker.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Endo, T. & Sanda, F. Molecular design of novel network polymers. Angew. Makromol. Chem. 240, 171–180 (1996).
Sanda, F. & Endo, T. Cationic equilibrium ring-opening polymerisation of bicyclic monomers and its application to chemical recycling of the new polymer material. Polym. Recycl. 3, 159–163 (1998).
Sanda, F. & Endo, T. Development of polymeric materials applicable to chemical recycling. Eco. Ind. 6, 18–26 (2001).
Kloxin, C. J., Scott, T. F., Adzima, B. J. & Bowman, C. N. Covalent adaptable networks (CANs): a unique paradigm in cross-linked polymers. Macromolecules 43, 2643–2653 (2010).
Maeda, T., Otsuka, H. & Takahara, A. Dynamic covalent polymers: reorganizable polymers with dynamic covalent bonds. Prog. Polym. Sci. 34, 581–604 (2009).
Endo, T. & Sudo, A. Development and application of novel ring-opening polymerizations to functional networked polymers. J. Polym. Sci. Part A: Polym. Chem. 47, 4847–4858 (2009).
Bergman, S. D. & Wudl, F. Mendable polymers. J. Mater. Chem. 18, 41–62 (2008).
Lehn, J. M. Dynamers: dynamic molecular and supramolecular polymers. Prog. Polym. Sci. 30, 814–831 (2005).
Mohr, G. J., Tirelli, N., Lohse, C. & Spichiger-Keller, U. Development of chromogenic copolymers for optical detection of amines. Adv. Mater. 10, 1353–1357 (1998).
Oh, K., Jeong, K. S. & Moore, J. S. m-Phenylene ethynylene sequences joined by imine linkages: dynamic covalent oligomers. J. Org. Chem. 68, 8397–8403 (2003).
Ono, T., Fujii, S., Nobori, T. & Lehn, J. M. Soft-to-hard transformation of the mechanical properties of dynamic covalent polymers through component incorporation. Chem. Commun. 46–48 (2007).
Chen, X., Dam, M. A., Ono, K., Mal, A., Shen, H., Nutt, S. R., Sheran, K. & Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 295, 1698–1702 (2002).
Binder, W. H. & Zirbs, R. Supramolecular polymers and networks with hydrogen bonds in the main- and side-chain. Adv. Polym. Sci. 207, 1–78 (2007).
ten Brinke, G., Ruokolainen, J. & Ikkala, O. Supramolecular materials based on hydrogen-bonded polymers. Adv. Polym. Sci. 207, 113–177 (2007).
Oku, T., Furusho, Y. & Takata, T. A concept for recyclable cross-linked polymers: Topologically networked polyrotaxane capable of undergoing reversible assembly and disassembly. Angew. Chem Int. Ed. Engl. 43, 966–969 (2004).
Bilig, T., Oku, T., Fursho, Y., Koyama, Y., Asai, S. & Takata, T. Polyrotaxane networks formed via rotaxanation utilizing dynamic covalent chemistry of disulfide. Macromolecules 41, 8496–8503 (2008).
Kohsaka, Y., Nakazono, K., Koyama, Y., Asai, S. & Takata, T. Size-complementary rotaxane cross-linking for the stabilization and degradation of a supramolecular network. Angew. Chem. Int. Ed. Engl. 50, 4872–4875 (2011).
Koyama, Y., Yoshii, T., Kohsaka, Y. & Takata, T. Photodegradable cross-linked polymer derived from a vinylic rotaxane cross-linker possessing aromatic disulfide axle. Pure Appl. Chem. 85, 835–842 (2013).
Arai, T., Jang, K., Koyama, Y., Asai, S. & Takata, T. Versatile supramolecular cross-linker: rotaxane cross-linker that directly endows vinyl polymers with movable cross-links. Chem. Eur. J. 19, 5917–5923 (2013).
Ashton, P. R., Baxter, I., Fyfe, M. C. T., Raymo, F. M., Spencer, N., Stoddart, J. F., White, A. J. P. & Williams, D. J. Rotaxane or pseudorotaxane? That is the question!. J. Am. Chem. Soc. 120, 2297–2307 (1998).
Tachibana, Y., Kawasaki, H., Kihara, N. & Takata, T. Sequential O- and N-acylation protocol for high-yield preparation and modification of rotaxanes: synthesis, functionalization, structure, and intercomponent interaction of rotaxanes. J. Org. Chem. 71, 5093–5104 (2006).
Makita, Y., Kihara, N. & Takata, T. Quantitative active transport in [2]Rotaxane using a one-shot acylation reaction toward the linear molecular motor. J. Org. Chem. 73, 9245–9250 (2008).
Tachibana, Y., Kihara, N., Furusho, Y. & Takata, T. Is the tert-Butyl group bulky enough to end-cap a pseudorotaxane with a 24-crown-8-ether wheel? Org. Lett. 6, 4507–4509 (2004).
Akae, Y., Okamura, H., Koyama, Y., Arai, T. & Takata, T. Selective synthesis of [3]Rotaxane consisting of size-complementary components and its stepwise deslippage. Org. Lett. 14, 2226–2229 (2012).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Nakazono, K. & Takata, T. Neutralization of a sec-ammonium group unusually stabilized by the “rotaxane effect”: synthesis, structure, and dynamic nature of a “free” sec-amine/crown ether-type rotaxane. Chem.-Eur. J. 16, 13783–13794 (2010).
Acknowledgements
This work was financially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 23245031).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Iijima, K., Kohsaka, Y., Koyama, Y. et al. Stimuli-degradable cross-linked polymers synthesized by radical polymerization using a size-complementary [3]rotaxane cross-linker. Polym J 46, 67–72 (2014). https://doi.org/10.1038/pj.2013.63
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2013.63