Abstract
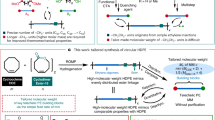
Herein, the extension of high-density polyethylene (PE) (HDPE) chains by formation of polypseudorotaxane structures with perpentylated pillar[5]arenes is reported. Melt mixing of polymeric chains of HDPEs and wheels of perpentylated pillar[5]arenes resulted in formation of polypseudorotaxane structures. The formation of the polypseudorotaxane structures led to extension of the HDPE chains, which dramatically increased the melting point of the HDPE from 126 to 152 °C. We also demonstrated molten-to-solid and solid-to-molten state transitions of HDPE based on the host–guest system. HDPE was melted at 140 °C, but changed to a solid upon addition of perpentylated pillar[5]arenes. Further addition of competitive guest 1,4-dibromobutane to the solid mixture induced a solid-to-molten state transition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yang, Y., Priyadarshani, N., Khamatnurova, T., Suriboot, J. & Bergbreiter, D. E. Polyethylene as a nonvolatile solid cosolvent phase for catalyst separation and recovery. J. Am. Chem. Soc. 134, 14714–14717 (2012).
Cheng, S., Chen, X., Hsuan, Y. G. & Li, C. Y. Reduced graphene oxide-induced polyethylene crystallization in solution and nanocomposites. Macromolecules 45, 993–1000 (2012).
Nakae, M., Uehara, H., Kanamoto, T., Zachariades, A. E. & Porter, R. S. Structure development upon melt drawing of ultrahigh molecular weight polyethylene: effect of prior thermal history. Macromolecules 33, 2632–2641 (2000).
Uehara, H., Kanamoto, T., Kawaguchi, A. & Murakami, S. Real-time X-ray diffraction study on two-stage drawing of ultra-high molecular weight polyethylene reactor powder above the static melting temperature. Macromolecules 29, 1540–1547 (1996).
Mandelkern, L. Crystallization of Polymers, (Cambridge University Press, Cambridge, UK, 2002).
Schulz, J. M. Polymer Crystallization, (Oxford University Press, New York, 2001).
Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T. & Nakamoto, Y. Para-bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 130, 5022–5023 (2008).
Ogoshi, T. Synthesis of novel pillar-shaped cavitands ‘pillar[5]arenes’ and their application for supramolecular materials. J. Incl. Phenom. Macrocycl. Chem. 72, 247–262 (2012).
Ogoshi, T. & Yamagishi, T. New synthetic host pillararenes: their synthesis and application to supramolecular materials. Bull. Chem. Soc. Jpn. 86, 312–332 (2013).
Ogoshi, T. & Yamagishi, T. Pillararenes: versatile synthetic receptors for supramolecular chemistry. Eur. J. Org. Chem. 15, 2961–2975 (2013).
Cragg, P. J. & Sharma, K. Pillar[5]arenes: fascinating cyclophanes with a bright future. Chem. Soc. Rev. 41, 597–607 (2012).
Xue, M., Yang, Y., Chi, X., Zhang, Z. & Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 45, 1294–1308 (2012).
Ogoshi, T., Demachi, K., Kitajima, K. & Yamagishi, T. Selective complexation of n-Alkanes with pillar[5]arene dimers in organic media. Chem. Commun. 47, 10290–10292 (2011).
Zhang, Z., Luo, Y., Chen, J., Dong, S., Yu, Y., Ma, Z. & Huang, F. Formation of linear supramolecular polymers that is driven by C-H···π interactions in solution and in the solid state. Angew. Chem. Int. Ed. 50, 1397–1401 (2011).
Strutt, N. L., Forgan, R. S., Spruell, J. M., Botros, Y. Y. & Stoddart, J. F. Monofunctionalized pillar[5]arene as a host for alkanediamines. J. Am. Chem. Soc. 133, 5668–5671 (2011).
Ogoshi, T., Aoki, T., Shiga, R., Iizuka, R., Ueda, S., Demachi, K., Yamafuji, D., Kayama, H. & Yamagishi, T. Cyclic host liquids for facile and high-yield synthesis of [2]rotaxanes. J. Am. Chem. Soc. 134, 20322–20325 (2012).
Ogoshi, T., Kitajima, K., Aoki, T., Fujinami, S., Yamagishi, T. & Nakamoto, T. Synthesis and conformational characteristics of alkyl-substituted pillar[5]arenes. J. Org. Chem. 75, 3268–3273 (2010).
Takahashi, T., Kawashima, H., Sugisawa, H. & Baba, T. 207Pb chemical shift thermometer at high temperature for magic angle spinning experiments. Solid State NMR 15, 119–123 (1999).
Takahashi, O., Kohno, Y. & Nishio, M. Relevance of weak hydrogen bonds in the conformation of organic compounds and bioconjugates: evidence from recent experimental data and high-level ab initio MO calculations. Chem. Rev. 110, 6049–6076 (2010).
Nishio, M. CH/π hydrogen bonds in crystals. Cryst. Eng. Commun. 6, 130–158 (2004).
Harada, A., Hashidzume, A., Yamaguchi, H. & Takashima, Y. Polymeric rotaxanes. Chem. Rev. 109, 5974–6023 (2009).
Wenz, G., Han, B. H. & Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 106, 782–817 (2006).
Li, J., Harada, A. & Kamachi, M. Formation of inclusion complexes of oligoethylene and its derivatives with α-cyclodextrin. Bull. Chem. Soc. Jpn 67, 2808–2818 (1994).
Sozzani, P., Comotti, A., Bracco, S. & Simonutti, R. Cooperation of multiple CH/π interactions to stabilize polymers in aromatic nanochannels as indicated by 2D solid state NMR. Chem. Commun. 768–769 (2004).
Comotti, A., Simonutti, R., Catel, G. & Sozzani, P. Isolated linear alkanes in aromatic nanochannels. Chem. Mater. 11, 1476–1483 (1999).
Sozzani, P., Comotti, A., Bracco, S. & Simonutti, R. A family of supramolecular frameworks of polyconjugated molecules hosted in aromatic nanochannels. Angew. Chem. Int. Ed. 43, 2792–2797 (2004).
Peersen, O. B., Wu, X., Kustanovich, I. & Smith, S. O. Variable-amplitude cross-polarization MAS NMR. J. Magn. Reson. A 104, 334–339 (1993).
Dixon, W. T., Schaefer, J. S., Sefcik, M. D., Stejskal, E. O. & McKay, R. A. Total suppression of sidebands in CPMAS C-13 NMR. J. Magn. Reson. 49, 341–345 (1982).
Bennett, A. E., Rienstra, C. M., Auger, M., Lakshmi, K. V. & Griffin, R. G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 103, 6951–6958 (1995).
Vinogradov, E., Madhu, P. K. & Vega, S. High-resolution proton solid-state NMR spectroscopy by phase-modulated Lee–Goldburg experiment. Chem. Phys. Lett. 314, 443–450 (1999).
Shu, X., Fan, J., Li, J., Wang, X., Chen, W., Jia, X. & Li, C. Complexation of neutral 1,4-dihalobutanes with simple pillar[5]arenes that is dominated by dispersion forces. Org. Biomol. Chem. 10, 3393–3397 (2012).
Acknowledgements
This work was partly supported by The Japan Securities Scholarship Foundation and Technology (MEXT), Japan and JSPS KAKENHI 23655210 (a Grant-in-Aid for Challenging Exploratory Research).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Ogoshi, T., Kayama, H., Aoki, T. et al. Extension of polyethylene chains by formation of polypseudorotaxane structures with perpentylated pillar[5]arenes. Polym J 46, 77–81 (2014). https://doi.org/10.1038/pj.2013.67
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2013.67
Keywords
This article is cited by
-
Supramolecular assemblies and polymer recognition based on polygonal and pillar-shaped macrocycles “pillar[n]arenes”
Polymer Journal (2023)
-
PJ ZEON Award for outstanding papers in Polymer Journal 2014
Polymer Journal (2015)
-
Molecular recognition and self-assembly of pillarenes
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2015)