Abstract
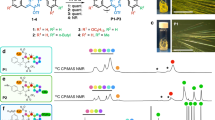
Multi-N-heterocycles could be a useful platform for constructing network structures via hydrogen bonds and for realizing catalyzed organic reactions. In addition, unique optical properties can be achieved by their incorporation into polymers. In this manuscript, the synthesis and characterization of an organic solvent-soluble polymer containing 1,3,4,6,9b-pentaazaphenalene are presented. From a series of optical measurements and comparative studies with model compounds, we investigated the electronic structures of polymer main chains containing the azaphenalene unit.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tokoro, Y., Yeo, H., Tanaka, K. & Chujo, Y. Synthesis of benzo[h]quinoline-based neutral pentacoordinate organosilicon complexes. Chem. Commun. 48, 8541–8543 (2012).
Tokoro, Y., Yeo, H., Tanaka, K. & Chujo, Y. Synthesis and tuning of optical properties of conjugated polymers involving benzo[h]quinoline-based neutral pentacoordinate organosilicon complexes in the main chain. Polym. Chem 4, 5237–5242 (2013).
Matsumoto, T., Tanaka, K. & Chujo, Y. Synthesis and optical properties of stable gallafluorene derivatives: investigation of their emission via triplet states. J. Am. Chem. Soc. 135, 4211–4214 (2013).
Tanaka, K., Murakami, M., Jeon, J.-H. & Chujo, Y. Enhancement of affinity in molecular recognition via hydrogen bonds by POSS-core dendrimer and its application for selective complex formation between guanosine triphosphate and 1,8-naphthyridine derivatives. Org. Biomol. Chem. 10, 90–95 (2012).
Lang, X., Chen, X. & Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 43, 473–486 (2014).
Underwood, G. R. Synthesis and reactions of some tetrahydroquinolizinium salts. Possible precursors to cycl[3.3.3]azine. J. Org. Chem 33, 1313–1317 (1968).
David, F. & Gough, T. T. Leaver, D. Heterocyclic compounds with bridgehead nitrogen atoms. Part V. Pyrido[2,1,6-de]quinolizines (Cycl[3.3.3]azines). J. Chem. Soc. Perkin Trans 1, 341–355 (1976).
Shaw, J. T., Balik, C. M., Holodnak, J. L. & Prem, S. Fused s-triazino heterocycles. IV. Electrophilic aromatic substitution reactions of some 1,3,4,6,9b-pentaazaphenalenes. J. Heterocyclic Chem 30, 127–130 (1976).
Balaban, A. T., Banciu, M. & Ciorba, V. Annulenes, Benzo-, Hetero-, Homo-Derivatives and Their Valence Isomers Vol. 3, (CRC Press, Boca Raton, FL, 1987).
Leupin, W. & Wirz, J. Low-lying electronically excited states of cycl[3.3.3]azine, a bridged 12π-perimeter. J. Am. Chem. Soc. 102, 6068–6075 (1980).
Boutique, J. P., Verbist, J. J., Fripiat, J. G., Delhalle, J., Pfister-Guillouzo, G. & Ashwell, G. J. 3,5,11,13-Tetraazacycl[3.3.3lazine: theoretical (ab initio) and experimental (X-ray and ultraviolet photoelectron spectroscopy) studies of the electronic structure. J. Am. Chem. Soc. 106, 4374–4378 (1984).
Rossman, M. A., Leonard, N. J., Urano, S. & LeBreton, P. R. Synthesis and valence orbital structures of azacycl[3.3.3lazines in a systematic series. J. Am. Chem. Soc 107, 3884–3890 (1985).
Gotou, H., Kurata, K., Tominaga, Y. & Matsuda, Y. Studies on quinolizine derivatives. 20. Syntheses of cyc1[3.3.3]azine derivatives. J. Org. Chem. 50, 4028–4032 (1985).
Leupin, W., Magde, D., Persy, G. & Wirz, J. 1,4,7-Triazacycl[3.3.3lazine: Basicity, photoelectron spectrum, photophysical properties. J. Am. Chem. Soc 108, 17–22 (1986).
Shahbaz, M., Urano, S., LeBreton, P. R., Rossman, M. A., Hosmane, R. S. & Leonard, N. J. Tri-s-triazine: synthesis, chemical behavior, and spectroscopic and theoretical probes of valence orbital Structure. J. Am. Chem. Soc. 106, 2805–2811 (1984).
Hosmane, R. S., Rossman, M. A. & Leonard, N. J. Synthesis and structure of tri-s-triazine. J. Am. Chem. Soc. 104, 5497–5499.
Shaw, U. T., Brotherton, C. E., Moon, R. W., Coffindaffer, T. W. & Miller, D. A. Fused s-triazino heterocycles. VIII. 1,3,4,6,9b-Pentaazaphenalenes. Reactions of a methyl and bromomethyl side chain. J. Heterocyclic Chem 18, 75–78 (1981).
Morita, Y., Suzuki, S., Sato, K. & Takui, T. Synthetic organic spin chemistry for structurally well-defined open-shell graphene fragments. Nat. Chem 3, 197–204 (2011).
Zheng, S., Lan, J., Khan, S. I. & Rubin, Y. Growth of protein crystals in hydrogels prevents osmotic shock. J. Am. Chem. Soc. 125, 5786–5789 (2003).
Morita, Y., Aoki, T., Fukui, K., Nakazama, S., Tamaki, K., Suzuki, S., Fuyuhiro, A., Yamamoto, K., Sato, K., Shiomi, D., Naito, A., Takui, T. & Nakasuji, K. A new trend in phenalenyl chemistry: a persistent neutral radical, 2,5,8-tri-tert-butyl-1,3-diazaphenalenyl, and the excited triplet state of the gable syn-dimer in the crystal of column motif. Angew. Chem. Int. Ed. 41, 1793–1796 (2002).
Morita, Y., Fukui, K., Suzuki, S., Aoki, T., Nakazawa, S., Tamaki, K., Fuyuhiro, A., Yamamoto, K., Sato, K., Shiomi, D., Naito, A., Takui, T. & Nakasuji, K. Syntheses and spin structures of 1,6-dithiapyrene derivatives having iminonitroxide or oxoverdazyl moieties. Polyhedron 22, 2199–2204 (2003).
Morita, Y., Suzuki, S., Fukui, K., Nakazawa, S., Kitagawa, H., Kishida, H., Okamoto, H., Naito, A., Sekine, A., Ohashi, Y., Shiro, M., Sasaki, K., Shiomi, D., Sato, K., Takui, T. & Nakasuji, K. Thermochromism in an organic crystal based on the co-existence of σ- and π-dimers. Nat. Mater. 7, 48–51 (2008).
Morita, Y., Suzuki, S., Fukui, K., Nakazawa, S., Sato, K., Shiomi, D., Takui, T. & Nakasuji, K. A synthetic study of metal complexes of coordinated neutral radicals based on an azaphenalenyl system. Polyhedron 22, 2215–2218 (2003).
Shaw, J. T., O'Connor, M. E., Allen, R. C., Westler, W. M. & Stefanko, B. D. Fused s-triazino heterocycles. II. 1,3,4,6,9b-Pentaazaphenalenes and 1,3,4,6,7,9b-hexaazaphenalene. J. Heterocycl. Chem. 11, 627–630 (1974).
Hosmane, R. S., Rossman, M. A. & Leonard, N. J. Synthesis and structure of tri-s-triazine. J. Am. Chem. Soc. 104, 5497–5499 (1982).
Shaw, J. T., Starkey, K. D., Pelliccione, D. J. & Barnhart, S. L. Fused s-triazino heterocycles. X. Displacement reactions of 7,9-dibromo-2-tribromomethyl-5-trichloromethyl-1,3,4,6,9b-pentaazaphenalene and 7,9-dibromo-2,5-bis(tribromomethyl)-1,3,4,6,9b-pentaazaphenalene. J. Heterocycl. Chem. 20, 1095–1097 (1983).
Laurent, G., Boutique, J. P., Evrard, G., Verbist, J. J. & Durant, F. Structure of 2,5-dimethyl-1,3,4,6-tetraazacycl[3.3.3]azine, C10H9N5. Acta Cryst C40, 2108–2109 (1984).
Devadoss, C., Bharathi, P. & Moore, J. S. Energy transfer in dendritic macromolecules: Molecular size effects and the role of an energy gradient. J. Am. Chem. Soc. 118, 9635–9644 (1996).
Kopelman, R., Shortreed, M., Shi, Z. Y., Tan, W., Xu, Z., Moore, J. S., Bar-Haim, A. & Klafter, J. Spectroscopic evidence for excitonic localization in fractal antenna supermolecules. Phys. Rev. Lett. 78, 1239–1242 (1997).
Swallen, S. F., Kopelman, R., Moore, J. S. & Devadoss, C. Dendrimer photoantenna supermolecules: energetic funnels, exciton hopping and correlated excimer formation. J. Mol. Struct. 485−486, 585–597 (1999).
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr. J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M. J., Knox, E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J, Dapprich, S, Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J. & Fox, D. J. Gaussian 09, revision B.01, (Gaussian, Inc., Wallingford CT, 2010).
Acknowledgements
This work was partially supported by the Mazda Foundation (for KT) and a Grant-in-Aid for Scientific Research on Innovative Areas, ‘New Polymeric Materials Based on Element-Blocks (No.2401)’ (24102013), provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Yeo, H., Hirose, M., Tanaka, K. et al. Construction of multi-N-heterocycle-containing organic solvent-soluble polymers with 1,3,4,6,9b-pentaazaphenalene. Polym J 46, 688–693 (2014). https://doi.org/10.1038/pj.2014.59
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2014.59