Abstract
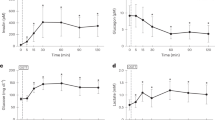
We previously observed young lambs to be more tolerant of hypoxia; compared with older lambs, they accumulate lactate at a slower rate during comparable reduction in cardiac output, and have a greater percent decrease in cardiac output before onset of systemic lactate accumulation. To determine the mechanism of lactic acidosis and the cause for this “tolerance,” we reduced cardiac output progressively in seven chronically catheterized conscious lambs (16.4 + 5.1 d) and measured hepatic and gastrointestinal (GI) blood flow (radioactive microspheres) and delivery, uptake, and extraction of lactate and O2. Hepatic O2 consumption declined proportionately below a critical hepatic O2 delivery (≅2 mL O2/min/kg), corresponding to the systemic O2 delivery associated with the onset of systemic lactate accumulation. As hepatic O2 delivery decreased below the critical value, there was initially net hepatic lactate uptake and then a change to net production when the O2 delivery decreased below ≅1 mL O2/min kg. The GI tract had net lactate production at rest, but surprisingly switched to lactate uptake as cardiac output decreased. The mechanism of lactic acidosis was failure of hepatic lactate uptake to increase despite increased hepatic lactate delivery, as reported in adults subjects. However, in contrast, there was “true” hepatic dysfunction and lactate production only at the lowest levels of cardiac output, after onset of systemic lactate accumulation. Moreover, we speculate that tolerance of young lambs to hypoxia is at least due to two factors: 1) hepatic lactate uptake is maintained beyond the “critical” O2 delivery and fall in hepatic O2 consumption, and 2) there is a switch to lactate uptake by the GI tract serving to buffer the lactate.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- GI:
-
gastrointestinal
- Hct:
-
hematocrit
- Hbo2:
-
fractional Hb oxygen saturation
References
Cain SM 1977 Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol 42: 228–234.
Simmons DH, Alpas AP, Tashkin DP, Coulson A 1978 Hyperlactatemia due to arterial hypoxemia or reduced cardiac output, or both. J Appl Physiol 45: 195–202.
Moss M, Moreau G, Lister G 1987 Oxygen transport and metabolism in the conscious lamb: the effects of hypoxemia. Pediatr Res 22: 177–183.
Fahey JT, Lister G 1987 Postnatal changes in critical cardiac output and O2 transport in conscious lambs. Am J Physiol 253:H100–H106.
Berry MN 1967 The liver and lactic acidosis. Proc R Soc Med 60: 52–54.
Schroder R, Gumpert JRW, Pluth JR, Elthringham WK, Jenny ME, Zollinger RM 1969 The role of the liver in the development of lactic acidosis in low flow states. Postgrad Med J 45: 566–570.
Tashkin DP, Goldstein PJ, Simmons DH 1972 Hepatic lactate uptake during decreased liver perfusion and hypoxemia. Am J Physiol 223: 968–974.
Samsel RW, Cherqui D, Pietrabissa A, Sanders WM, Roncella M, Emond JC, Schumacker PT 1991 Hepatic oxygen and lactate extraction during stagnant hypoxia. J Appl Physiol 70: 186–193.
Schlichtig R, Klions HA, Kramer DJ, Nemoto EM 1992 Hepatic dysoxia commences during O2 supply dependence. J Appl Physiol 72: 1499–1505.
Fahey JT, Lister G 1989 Response to low cardiac output: developmental differences in metabolism during oxygen deficit and recovery in lambs. Pediatr Res 26: 180–187.
Gleason CA, Roman C, Rudolph AM 1985 Hepatic O2 consumption, lactate uptake, and glucose production in neonatal lambs. Pediatr Res 19: 1235–1239.
Harrison F, Linzell J, Paterson J 1972 Oxygen consumption by the liver of the conscious sheep. J Anat 111: 330
Fahey JT, Lister G 1985 A simple method for reducing cardiac output in the conscious lamb. Am J Physiol 249:H188–H192.
Edelstone DI, Holzman IR 1981 Gastrointestinal tract O2 uptake and regional blood flows during digestion in conscious newborn lambs. Am J Physiol 241: G289–G293.
Lister G, Hoffman JIE, Rudolph AM 1974 Oxygen uptake in infants and children: a simple method of measurement. Pediatrics 53: 656–662.
Heymann MA, Payne BD, Hoffman JIE, Rudolph AM 1977 Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20: 55–79.
Delaney JP 1969 Arteriovenous anastomotic blood flow in the mesenteric organs. Am J Physiol 216: 1556–1561.
Greenway CV, Murthy VS 1972 Effects of vasopressin and isoprenaline infusions on the distribution of blood flow in the intestine; criteria for the validity of microsphere studies. Br J Pharmacol 46: 177–188.
Botti JJ, Edelstone DI, Caritis SN, Mueler-Heubach E 1982 Portal venous blood flow distribution to liver and ductus venosus in newborn lambs. Am J Obstet Gynecol 144: 303–308.
Edelstone DI, Holzman IR 1981 Oxygen consumption by the gastrointestinal tract and liver in conscious new born lambs. Am J Physiol 240:G297–G304.
Samsel RW, Schumacker PT 1988 Determination of the critical O2 delivery from experimental data: sensitivity to error. J Appl Physiol 64: 2074–2082.
Edelstone DI, Paulone ME, Holzman IR 1984 Hepatic oxygenation during arterial hypoxemia in neonatal lambs. Am J Obstet Gynecol 150: 513–518.
Lutz J, Henrich H, Bauereisen E 1975 Oxygen supply and uptake in the liver and the intestine. Pflugers Arch 360: 7–15.
Metcalfe HK, Monson JP, Welch SG, Cohen RD 1986 Inhibition of lactate removal by ketone bodies in rat liver. J Clin Invest 78: 743–747.
Monson JP, Smith JA, Cohen RD, Iles RA 1982 Evidence for a lactate transporter in the plasma membrane of the rat hepatocyte. Clin Sci 62: 411–420.
Fafournoux P, Demigne C, Christian R 1985 Carrier-medicated uptake of lactate in rat hepatocytes. J Biol Chem 260: 292–299.
Naylor JM, Kronfeld DS, Freeman DE, Richardson D 1984 Hepatic and extrahepatic lactate metabolism in sheep: effects of lactate loading and pH. Am J Physiol 247: E747–E755.
Iles RA, Cohen RD, Baron PG 1981 The effect of combined ischaemia and acidosis on lactate uptake and gluconeogenesis in the perfused rat liver. Clin Sci 60: 537–542.
Iles RA, Cohen RD, Baron PG, Smith JA, Henderson RM 1981 The effect of adrenaline on hepatic lactate uptake in the acidotic partially ischaemic rat liver. Clin Sci 60: 543–548.
Sidi D, Kuipers JR, Teitel D, Heymann MA, Rudolph AM 1983 Developmental changes in oxygenation and circulatory responses to hypoxemia in lambs. Am J Physiol 245:H674–H682.
Robin E 1978 Overview, dysoxia-abnormalities of tissue oxygen use. In: Lenfant C (ed) Extrapulmonary Manifestations of Respiratory Disease. Marcel Dekker, New York, pp 3–12.
Adams RP, Cain SM 1983 Total and hindlimb oxygen deficit and “repayment” in hypoxic anesthetized dogs. J Appl Physiol 55: 913–922.
Hochachka P, Guppy M 1987 Metabolic Arrest and the Control of Biological Time. Harvard University Press, Cambridge, MA, pp 10–35.
Clark M, Filsell O, Jarrett I 1976 Gluconeogenesis in isolated intact lamb liver cells. Biochem J 156: 671–680.
Armentano L 1992 Ruminant hepatic metabolism of volatile fatty acids, lactate and pyruvate. J Nutr 122: 838–842.
Acknowledgements
The authors thank Andrea O. Ray, Anthony Battelli, and Vincent Santucci for their expert technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by a Child Health Research Grant from the Charles H. Hood Foundation. D.J.S. was supported by a National Heart, Lung, and Blood Institute, Training Grant HL07272. D.I.E. was supported by National Institutes of Health Grant HD19092. G.L. is an Established Investigator of the American Heart Association.
APPENDIX
APPENDIX
Calculations. Oxygen contents. Aortic (a), pulmonary arterial (mixed venous, v), portal venous (pv), and hepatic venous (hv) blood O2 contents (Cao2, Cvo2, Cpvo2, Chvo2, in mL O2/dL) were calculated from the respective Po2 (mm Hg), Hbo2 (fractional), and Hb (g/dL) as: Equation where 0.003 (mL O2/dL plasma mm Hg) is the Bunsen solubility coefficient for O2 in plasma at 37 °C. x = a, v, pv, hv.

Cardiac output. Qt, in mL/min/kg, was calculated using the Fick equation, from whole body O2 consumption (Vo2 in mL/min kg), Cao2, and Cvo2 as: Equation

Systemic O2 transport. Systemic O2 transport(SOT, in mL O2/min/kg) was calculated from its definition:Equation

Hepatic O2 delivery. Oxygen delivered to the liver (mL O2/min) was calculated as the sum of portal vein and hepatic artery contributions, with blood flows (Qpv, Qha, Qh) in mL/min:Equation

Hepatic O2 consumption. Oxygen consumption by the liver (mL O2/min) was calculated by: Equation where Qh = total liver blood flow = Qpv + Qha.

Hepatic O2 extraction. Hepatic O2 extraction was calculated as the quotient of hepatic O2 consumption and hepatic O2 delivery.
Hepatic lactate delivery. The lactate delivered to the liver(mmol/min) was calculated as for O2 delivery, substituting [L] (plasma lactate concentration, in mmol/L plasma) for O2 content.Equation where PCV = packed cell volume = Hct/100; this assumes that packed cell volume is the same in portal and arterial vessels.

Net hepatic lactate uptake (mmol/min).Equation This equation yields uptake of lactate from plasma and assumes that this is similar to uptake from blood because erythrocyte lactate equilibrates slowly with plasma(27).

Actual hepatic lactate extraction ratio.Equation We noted that the hepatic artery flows are a small percentage of the total liver blood flow (<10%) and the majority of hepatic blood flow at all levels of cardiac output was predominately portal flow (i.e. Qha ≪ Qpv; Qh ≅ Qpv). The formula for hepatic lactate extraction is greatly simplified if Qha is assumed to be zero and Qpv= Qh. Making these substitutions yields:

Estimated hepatic lactate extraction ratio.Equation As seen, all flow terms cancel and the estimation requires only measurement of lactate in the portal and hepatic veins. To examine the validity of this assumption, the estimated hepatic lactate extraction is plotted as a function of the actual hepatic lactate extraction in Figure 6. As seen, the linear regression analysis shows an excellent correlation (r = 0.99) with a slope close to one (0.99) and a SEM of the estimate of 0.036. The estimated lactate extraction is obviously a much easier measure to make, because it requires no measurement of blood flow, but only the ability to sample from the hepatic vein and portal vein. This estimation is accurate in the lamb because portal blood flow accounts for 90% of the total hepatic blood flow. The estimation may be less valid in species who have a larger proportion of the hepatic blood flow supplied by the hepatic artery, for example in man or pigs where the hepatic artery supplies approximately one-third of the hepatic blood flow. In the lamb, this estimation can be useful for assessing lactate extraction during times or studies where flow measurements are not possible or are limited in number.

Net GI O2 consumption.Equation where GI includes stomach, small bowel, large bowel, and spleen.

Net GI lactate uptake. Equation

GI lactate extraction. Equation

Rights and permissions
About this article
Cite this article
Fahey, J., Lister, G., Sanfilippo, D. et al. Hepatic and Gastrointestinal Oxygen and Lactate Metabolism during Low Cardiac Output in Lambs. Pediatr Res 41, 842–851 (1997). https://doi.org/10.1203/00006450-199706000-00008
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199706000-00008