Abstract
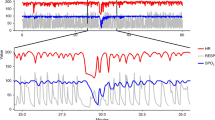
We introduce the sample asymmetry analysis (SAA) and illustrate its utility for assessment of heart rate characteristics occurring early in the course of neonatal sepsis and systemic inflammatory response syndrome (SIRS). Conceptually, SAA describes changes in the shape of the histogram of RR intervals that are caused by reduced accelerations and/or transient decelerations of heart rate. Unlike other measures of heart rate variability, SAA allows separate quantification of the contribution of accelerations and decelerations. The application of SAA is exemplified by a study comparing 50 infants, who experienced a total of 75 episodes of sepsis and SIRS, with 50 control infants. The two groups were matched by birth weight and gestational age. RR intervals were recorded for all infants throughout their course in the Neonatal Intensive Care Unit. The sample asymmetry of the RR intervals increased in the 3–4 d preceding sepsis and SIRS, with the steepest increase in the last 24 h, from a baseline value of 3.3 (SD = 1.6) to 4.2 (SD = 2.3), p = 0.02. After treatment and recovery, sample asymmetry returned to its baseline value of 3.3 (SD = 1.3). The difference between sample asymmetry in health and before sepsis and SIRS was mainly due to fewer accelerations than to decelerations. Compared with healthy infants, infants who experienced sepsis had similar sample asymmetry in health, and elevated values before sepsis and SIRS (p = 0.002). We conclude that SAA is a useful new mathematical technique for detecting the abnormal heart rate characteristics that precede neonatal sepsis and SIRS.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- SAA:
-
sample asymmetry analysis
- HRC:
-
heart rate characteristics
- BW:
-
birth weight
- GA:
-
gestational age
- HR:
-
heart rate
- SIRS:
-
systemic inflammatory response syndrome
REFERENCES
Ventura SJ, Martin JA, Mathews TJ, Clarke SC 1996 Advance report of final natality statistics. Mon Vital Stat Rep 44: 1–88
Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR 1996 Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 129: 63–71
Gray JE, Richardson DK, McCormick MC, Goldmann DA 1995 Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics 95: 225–230
Members of the ACCP/SCCM Consensus Conference Committee, American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference 1992 Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864–874
Bone RC, Grodzin CJ, Balk RA 1997 Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 112: 235–243
Gladstone IM, Ehrenkrantz RA, Edberg SC, Baltimore RS 1990 A ten-year review of neonatal sepsis and comparison with the previous fifty year experience. Pediatr Infect Dis J 9: 819–825
Moro ML, DeToni A, Stolfi I, Carrieri MP, Braga M, Zunin C 1996 Risk factors for nosocomial sepsis in newborn infants and intermediate care units. Eur J Pediatr 155: 315–322
Escobar GJ 1999 The neonatal “sepsis work-up”: personal reflections on the development of an evidence-based approach toward newborn infections in a managed care organization. Pediatrics 103: 360–373
Gerdes JS, Polin RA 1987 Sepsis screen in neonates with evaluation of plasma fibronectin. Pediatr Infect Dis J 6: 443–446
Kaftan H, Kinney JS 1998 Early onset neonatal bacterial infections. Semin Perinatol 22: 15–24
Aronson MD, Bor DH 1987 Blood cultures. Ann Intern Med 106: 246–253
Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA 1997 Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J 16: 381–385
Griffin MP, Moorman JR 2001 Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 107: 97–104
Lake DE, Richman JS, Griffin MP, Moorman JR 2002 Sample entropy analysis of neonatal heart rate variability. Am J Physiol 283: R789–R797
Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke WL 1998 Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 21: 1870–1875
Kovatchev BP, Straume M, Cox DJ, Farhy LS 2001 Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes. J Theor Med 3: 1–10
Chang KL, Monahan KJ, Griffin MP, Lake DE, Moorman JR 2001 Evaluation of frequency domain methods for detection of sinus arrhythmia in neonatal heart rate time series. Ann Biomed Eng 29: 764–774
Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Lake DE, Moorman JR . Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res 53: 920–926
Kleiger RE, Miller JP, Bigger JT, Moss AJ 1987 Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262
Richman JS, Moorman JR 2000 Physiological time series analysis using approximate entropy and sample entropy. Am J Physiol 278: H2039–H2049
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Institutes of Health grants R01-DK51562 and GM64640; the American Heart Association, Mid-Atlantic Consortium; Children's Medical Center Research Fund, University of Virginia; W.S. Paley Foundation; Virginia's Center for Innovative Technology; and Medical Decision Networks, Charlottesville, VA.
Potential for conflict of interest: Medical Decision Networks of Charlottesville, VA, which supplied partial funding for this study, has a license to market technology related to heart rate characteristics (HRC) monitoring of newborn infants. As of the submission date of the final version of the article, none of the authors had received consultants' fees or owned equity in Medical Decision Networks or related companies. However, Drs. Griffin and Moorman have been offered an equity share of a new company, Medical Predictive Systems Corporation, that owns the HRC technology license.
APPENDIX: SAMPLE ASYMMETRY OF A RANDOM VARIABLE
APPENDIX: SAMPLE ASYMMETRY OF A RANDOM VARIABLE
To define sample asymmetry of a random variables we first introduce the following:
Weighting functions.
Let ξ be a random variable with values in its sampling space X and unspecified distribution, and let μ ∈X be a point within the sampling space X. For any x ∈X we define a weighting function w (x; α) = (x − μ) α, where α > 0 is a parameter describing the degree of weighting of deviations from the reference point μ. For example, if α = 1, deviations from μ will receive linearly increasing weights, whereas if α = 2, deviations from μ will receive quadratically increasing weights. Note that the weighting parameter α could be selected in various applications to be any positive, including noninteger, number. A number smaller than 1 will result in slower than linear increase of weights, a number greater than 2 will result in a faster than quadratic increase of weights. Further, we define separate weighting for left and right deviations of ξ from its reference point μ as follows: 1) Left-weighting function:w1(x; α) =w (x; α) whenever x < μ and 0 otherwise, and 2) right-weighting function:w2(x; β) =w (x; β) whenever x ≥ μ and 0 otherwise, where the parameter β, similarly to α, describes the degree of weighting of deviation to the right of the reference point. To add flexibility to this model, we allow for different degree of weighting to the left (α) and to the right (β) from the reference point μ. In many applications, the left and right weightings could be equal (see Fig. 1).
Defining sample asymmetry of a random variable.
Let x1, x2, …xn be a sample of n observations on ξ. Given this sample, we define two quantities representing the sum of the weighted deviations to the left and to the right from the reference point μ as follows:

It is now clear that if α = β, and the sample x1, x2, …xn is approximately symmetric with respect to the reference point, then R1 will be approximately equal to R2. If the sample is asymmetric with larger and/or more frequent deviations to the right from the reference point μ, then R2 will be greater than R1. Inversely, if the sample is asymmetric with larger and/or more frequent deviations to the left from the reference point μ, then R1 will be greater than R2.
Definition: The ratio

represents the sample asymmetry of the random variable.
The following properties are pertinent to the applications of sample asymmetry:
(a) If α = β, when the sample x1, x2, …xn is approximately symmetric with respect to the reference point, then R(α, β) will be approximately equal to 1. Values greater than 1 will indicate larger and/or more frequent deviations to the right from the reference point μ, whereas values less than 1 will indicate larger and/or more frequent deviations to the left.
(b) The sensitivity of the ratio R(α, β) to left and right deviations from the reference point can be controlled through separate adjustment of the parameters α and β.
(c) R1(α) and R2(β) can be used separately as estimates of the absolute weighted mass of the distribution of ξ; with respect to its reference point μ.
(d) The reference point μ can be the empirical mean of the random variable ξ, e.g.

the median of ξ, or any other theoretically or practically relevant number. The choice of a reference point could be critical for the subsequent data analyses. The median of the distribution is an obvious initial reference point because the median, as opposed to other statistics such as the mean, is not affected by outliers or large deviations in the data. This is particularly important in the analysis of RR intervals where the goal is to quantify spikes in the data.
(e) A quadratic weighting, that is, α = β = 2, has special properties that set it apart from other parameter configurations. First, the weighted deviations R1(α) and R2(β) are exactly the squares of the classical Euclidean distances of the sample measured to the left and to the right from the reference point. Second, if the reference point μ is the mean (or the median) of the distribution, then, under the null hypothesis that the distribution is normal, the sample asymmetry R(2, 2) will have an F distribution with (n/2–1;n/2–1) degrees of freedom (n/2–1/2;n/2–3/2 if n is an odd number). This property gives a straightforward statistical test for symmetry of a single data sample. Thus, we recommend the use of a quadratic weighting unless a particular data sample proves that another parameter combination is more appropriate.
Rights and permissions
About this article
Cite this article
Kovatchev, B., Farhy, L., Cao, H. et al. Sample Asymmetry Analysis of Heart Rate Characteristics with Application to Neonatal Sepsis and Systemic Inflammatory Response Syndrome. Pediatr Res 54, 892–898 (2003). https://doi.org/10.1203/01.PDR.0000088074.97781.4F
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000088074.97781.4F
This article is cited by
-
Sample entropy correlates with intraventricular hemorrhage and mortality in premature infants early in life
Pediatric Research (2024)
-
Vital signs as physiomarkers of neonatal sepsis
Pediatric Research (2022)
-
Fetal heart rate variability is a biomarker of rapid but not progressive exacerbation of inflammation in preterm fetal sheep
Scientific Reports (2022)
-
Discovery of signatures of fatal neonatal illness in vital signs using highly comparative time-series analysis
npj Digital Medicine (2022)
-
The principles of whole-hospital predictive analytics monitoring for clinical medicine originated in the neonatal ICU
npj Digital Medicine (2022)