Abstract
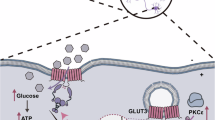
The intestine is a major site of amino acid metabolism, especially in neonates. The energy needed for the metabolic processes in neonatal animals is derived from dietary glucose and amino acids. No data are available showing that dietary amino acids function as intestinal fuel source in human neonates as well. We hypothesized that preterm infants show a high splanchnic first-pass glutamate metabolism and the primary metabolic fate of glutamate is oxidation. Five preterm infants (birth weight 1.2 ± 0.2 kg, gestational age 29 ± 1 wk) were studied by dual tracer ([U-13C]glutamate and [D3]glutamate) techniques on two study days (within postnatal d 14–19). Splanchnic and whole-body glutamate kinetics were assessed by plasma isotopic enrichment of [U-13C]glutamate and [D3]glutamate and breath 13CO2 enrichment. Fractional first-pass glutamate uptake was 77 ± 18% on d 1, and 70 ± 7% on d 2, mean 74 ± 13%. Almost all (86 ± 7%) of the glutamate used in the first pass is directed toward oxidation. There is a high splanchnic fractional first-pass uptake and a high oxidation rate of glutamate in preterm infants. Glutamate is an important source of energy for the splanchnic tissues in preterm infants receiving full enteral feeding.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APE:
-
atom percent excess
- GSH:
-
glutathione
- ORS:
-
oral rehydration solution
References
Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG 1998 Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr 128: 606–614
van Goudoever JB, Stoll B, Henry JF, Burrin DG, Reeds PJ 2000 Adaptive regulation of intestinal lysine metabolism. Proc Natl Acad Sci U S A 97: 11620–11625
Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ 1999 Substrate oxidation by the portal drained viscera of fed piglets. Am J Physiol 277: E168–E175
Hoerr RA, Matthews DE, Bier DM, Young VR 1991 Leucine kinetics from [2H3]- and [13C]leucine infused simultaneously by gut and vein. Am J Physiol 260: E111–E117
Matthews DE, Marano MA, Campbell RG 1993 Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol 264: E109–E118
Beaufrere B, Fournier V, Salle B, Putet G 1992 Leucine kinetics in fed low-birth-weight infants: importance of splanchnic tissues. Am J Physiol 263: E214–E220
van der Schoor SR, Reeds PJ, Stellaard F, Wattimena JD, Sauer PJ, Buller HA, van Goudoever JB 2004 Lysine kinetics in preterm infants: the importance of enteral feeding. Gut 53: 38–43
Reeds PJ, Burrin DG, Stoll B, Jahoor F, Wykes L, Henry J, Frazer ME 1997 Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am J Physiol 273: E408–E415
Reeds PJ, Burrin DG, Jahoor F, Wykes L, Henry J, Frazer EM 1996 Enteral glutamate is almost completely metabolized in first pass by the gastrointestinal tract of infant pigs. Am J Physiol 270: E413–E418
van der Schoor SR, van Goudoever JB, Stoll B, Henry JF, Rosenberger JR, Burrin DG, Reeds PJ 2001 The pattern of intestinal substrate oxidation is altered by protein restriction in pigs. Gastroenterology 121: 1167–1175
Matthews DE, Marano MA, Campbell RG 1993 Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol 264: E848–E854
Battezzati A, Brillon DJ, Matthews DE 1995 Oxidation of glutamic acid by the splanchnic bed in humans. Am J Physiol 269: E269–E276
Rautonen J, Makela A, Boyd H, Apajasalo M, Pohjavuori M 1994 CRIB and SNAP: assessing the risk of death for preterm neonates. Lancet 343: 1272–1273
van der Schoor SR, de Koning BA, Wattimena DL, Tibboel D, van Goudoever JB 2004 Validation of the direct nasopharyngeal sampling method for collection of expired air in preterm neonates. Pediatr Res 55: 50–54
Riedijk MA, Voortman G, van Goudoever JB 2005 Use of [13C]bicarbonate for metabolic studies in preterm infants: intragastric versus intravenous administration. Pediatr Res 58: 861–864
Waterlow JC 1967 Lysine turnover in man measured by intravenous infusion of L-[U-14C]lysine. Clin Sci 33: 507–515
Tserng KY, Kalhan SC 1983 Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol 245: E308–E311
van der Schoor SR, Stoll B, Wattimena DL, Buller HA, Tibboel D, Burrin DG, van Goudoever JB 2004 Splanchnic bed metabolism of glucose in preterm neonates. Am J Clin Nutr 79: 831–837
Haÿs SP, Ordonez JM, Burrin DG, Sunehag AL 2007 Dietary glutamate is almost entirely removed in its first pass through the splanchnic bed in premature infants. Pediatr Res 62: 353–356
Windmueller HG, Spaeth AE 1975 Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys 171: 662–672
Neame KD, Wiseman G 1957 The transamination of glutamic and aspartic acids during absorption by the small intestine of the dog in vivo. J Physiol 135: 442–450
Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R 2005 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 41: S1–S87
Sulkers EJ, von Goudoever JB, Leunisse C, Wattimena JL, Sauer PJ 1992 Comparison of two preterm formulas with or without addition of medium-chain triglycerides (MCTs). I: Effects on nitrogen and fat balance and body composition changes. J Pediatr Gastroenterol Nutr 15: 34–41
Aw TY, Williams MW 1992 Intestinal absorption and lymphatic transport of peroxidized lipids in rats: effect of exogenous GSH. Am J Physiol 263: G665–G672
van Goudoever JB, Sulkers EJ, Chapman TE, Carnielli VP, Efstatopoulos T, Degenhart HJ, Sauer PJ 1993 Glucose kinetics and glucoregulatory hormone levels in ventilated preterm infants on the first day of life. Pediatr Res 33: 583–589
Acknowledgements
The authors thank Ko Hagoort for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sophia Foundation for Medical Research (grant 417), The Netherlands, Numico Foundation, Wageningen, The Netherlands, and Ajinimoto, Japan.
Appendix 1
Appendix 1
Glutamate flux during i.v. infusion was calculated as follows (7):
MATH

where QIV is the flux of the i.v. infused tracer [μmol/(kg · h)], iglu_iv is the i.v. glutamate infusion rate [μmol/(kg · h)], and IEi and IEp are the isotopic enrichments (mol% excess) of i.v. administered [U-13C or D3]glutamate in the infusate or in plasma at steady state, respectively.
The flux for the intragastrically administered tracer was calculated as follows:
MATH

where QIG is the flux of the intragastrically infused tracer [μmol/(kg · h)], iglu_ig is the intragastric glutamate infusion rate (μmol/(kg · h), and IEi, and IEp are the isotopic enrichments (mol% excess) of intragastrically administered [U-13C or D3]glutamate in the infusate or in plasma at steady state, respectively.
First-pass glutamate uptake was calculated as:
MATH

where U is the first-pass glutamate uptake μmol/(kg · h)], QIG is the flux of the intragastrically administered glutamate tracer [μmol/(kg · h)], QIV is the flux of the i.v. administered glutamate tracer [μmol/(kg · h)], and I is the intake of enteral glutamate [μmol/(kg · h)].
Whole-body CO2 production was estimated as:
MATH

where iB is the infusion rate of [13C]sodium bicarbonate [(μmol/(kg · h)], IEiB is the enrichment (mol% excess) of [13C]bicarbonate in the bicarbonate infusate, and IEB is the 13CO2 enrichment at steady state during the labeled bicarbonate infusion (mol% excess). Splanchnic and whole-body glutamate oxidation rates were determined under the assumption that CO2 production during [13C]bicarbonate infusion equals the labeled glutamate tracer infusion. Assuming a constant CO2 production rate during the 7 h of infusion, it is not necessary to use a correction factor for retention of [13C]bicarbonate in the body (25).
Glutamate oxidation was calculated by multiplying recovery of [13C]glutamate in expiratory air with the rate of appearance of glutamate (7,25). The fraction of glutamate oxidized using the enrichment of the i.v. [U-13C]glutamate infusion on study d 1 and intragastric [U-13C]glutamate on study d 2 was calculated as:
MATH

where IEglu and IEB are 13CO2 enrichments (mol% excess) in expired breath at steady state during i.v. (d 1) or intragastric (d 2) [U-13C]glutamate and [13C]bicarbonate infusion. The iglu is multiplied by a factor of 5 to account for the number of labeled C atoms.
Whole-body glutamate oxidation (day 1) was calculated as:
MATH

Rights and permissions
About this article
Cite this article
Riedijk, M., de Gast-Bakker, DA., Wattimena, J. et al. Splanchnic Oxidation Is the Major Metabolic Fate of Dietary Glutamate in Enterally Fed Preterm Infants. Pediatr Res 62, 468–473 (2007). https://doi.org/10.1203/PDR.0b013e31813cbeba
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e31813cbeba
This article is cited by
-
Comments on “Assessment of changes in the liver of pregnant female rats and their fetuses following monosodium glutamate administration” by Gad El-Hak et al., http://doi.org/10.1007/s11356-021–13,557-7
Environmental Science and Pollution Research (2022)
-
Effects of adding sodium dichloroacetate to low-protein diets on nitrogen balance and amino acid metabolism in the portal-drained viscera and liver of pigs
Journal of Animal Science and Biotechnology (2020)
-
Glutamate metabolism in a human intestinal epithelial cell layer model
Amino Acids (2020)
-
Glutamate: a truly functional amino acid
Amino Acids (2013)