Abstract
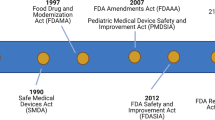
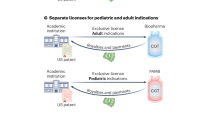
In November 2014, the American Academy of Pediatrics convened key stakeholders to discuss the feasibility of accelerating children’s medical advances by creating an independent global Pediatric Clinical Trials Network. The Forum identified challenges posed by the US and global clinical trial systems regarding testing and disseminating drugs and devices for pediatric patients. Stakeholders mapped a vision to improve the safety and efficacy of pediatric drugs, biological products, and medical devices by creating a global Pediatric Clinical Trials Network. Such a Network would act as a central infrastructure for pediatric subspecialties and enable dedicated staff to provide clinical research sites with scientific, medical, and operational support. A Network would facilitate development and availability of innovative, high-quality therapies to extend and enhance the lives of neonates, infants, children, adolescents, and young adults. Participants expressed strong interest in forming such a Network, since drugs and devices still come to market without adequate pediatric indications—particularly in neonatology and rare diseases. Participants developed a Consensus Statement expressing their shared vision for a Network: Attendees of the Pediatric Clinical Trials Stakeholder Forum resolved to establish a Global Pediatric Clinical Trials Network and are committed to engage in the work to create and sustain it.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Food and Drug Administration. Pediatric studies – annual status summary, no date. (www.fda.gov/downloads/scienceresearch/specialtopics/pediatrictherapeuticsresearch/ucm308465.pdf.)
Food and Drug Administration. Consumer updates: giving drugs to children, 2015. (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm164427.htm.)
Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 2006;5:703–18.
Food and Drug Administration. Formulations: pediatric formulations in accordance with sections 505A(f)(6)(D) and 505B(f)(6)(F) of the Federal Food, Drug, and Cosmetic Act, as amended by the FDA Amendments Act (FDAAA), 2014. (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049872.htm.)
National Library of Medicine. NITROPRESS®: Sodium Nitroprusside Injection, solution, concentrate, 2014. (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bf27f0a9-e64b-492a-b08f-2448bf097988.)
The Pink Sheet. Karlin S. FDA’s Woodcock prioritizes trial networks as drug development solution, 2014. (http://www.focr.org/5-7-2014-pink-sheet-fda’s-woodcock-prioritizes-trial-networks-drug-development-solution.)
Acknowledgements
We acknowledge and appreciate the hard work of the members of the Planning Committee, who provided input into the organization and process for the Stakeholder Forum. They participated in the event itself, where they not only served as content experts but also contributed to the meeting discussions, workgroups, ideas, and outcomes.
Author information
Authors and Affiliations
Consortia
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Bogue, C., DiMeglio, L., Maldonado, S. et al. Special article: 2014 Pediatric Clinical Trials Forum. Pediatr Res 79, 662–669 (2016). https://doi.org/10.1038/pr.2015.255
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.255
This article is cited by
-
The future of pediatric research: European perspective
Pediatric Research (2017)
-
Moving toward a paradigm shift in the regulatory requirements for pediatric medicines
European Journal of Pediatrics (2016)