Abstract
Background
Cystein-rich protein 61 (Cyr61/CCN1) is a member of the CCN family of matricellular proteins that has an important role in tissue development and remodeling. However, the role of CCN1 in the pathogenesis of bronchopulmonary dysplasia (BPD) is unknown. Accordingly, we have investigated the effects of CCN1 on a hyperoxia-induced lung injury model in neonatal rats.
Methods
In experiment 1, newborn rats were randomized to room air (RA) or 85% oxygen (O2) for 7 or 14 days, and we assessed the expression of CCN1. In experiment 2, rat pups were exposed to RA or O2 and received placebo or recombinant CCN1 by daily intraperitoneal injection for 10 days. The effects of CCN1 on hyperoxia-induced lung inflammation, alveolar and vascular development, vascular remodeling, and right ventricular hypertrophy (RVH) were observed.
Results
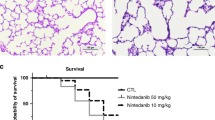
In experiment 1, hyperoxia downregulated CCN1 expression. In experiment 2, treatment with recombinant CCN1 significantly decreased macrophage and neutrophil infiltration, reduced inflammasome activation, increased alveolar and vascular development, and reduced vascular remodeling and RVH in the hyperoxic animals.
Conclusion
These results demonstrate that hyperoxia-induced lung injury is associated with downregulated basal CCN1 expression, and treatment with CCN1 can largely reverse hyperoxic injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Van Marter LJ . Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2009;14:358–66.
Bhandari A, Bhandari V . Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–73.
Wright CJ, Kirpalani H . Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 2011;128:111–26.
Jobe AJ, Bancalari E . Bronchopulmonary dysplasia. Am J Resp Crit Care Med 2001;163:1723–9.
Coalson JJ, Winter V, deLemos RA . Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Resp Crit Care Med 1995;152:640–6.
Perbal B . CCN proteins: multifunctional signalling regulators. Lancet 2004;363:62–4.
Chaqour B, Goppelt-Struebe M . Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J 2006;273:3639–49.
Allen JT, Knight RA, Bloor CA et al. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am Respir Cell Mol Biol 1999;21:693–700.
Sato S, Nagaoka T, Hasegawa M et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol 2000;27:149–54.
Wu S, Capasso L, Lessa A et al. High tidal volume ventilation activates Smad2 and upregulates expression of connective tissue growth factor in newborn rat lung. Pediatr Res 2008;63:245–50.
Chen CM, Wang LF, Chou HC et al. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 2007;62:128–33.
Wu S, Platteau A, Chen S et al. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am Respir Cell Mol Biol 2010;42:552–63.
Chen S, Rong M, Platteau A et al. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2011;300:L330–40.
Alapati D, Rong M, Chen S et al. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am Respir Cell Mol Biol 2011;45:1169–77.
Jun J-I, Lau LF . Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 2011;10:945–63.
Jun JI, Kim KH, Lau LF . The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun 2015;6:7386.
Kim KH, Chen CC, Monzon RI et al. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 2013;33:2078–90.
Leu SJ, Lam SC, Lau LF . Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 2002;277:46248–55.
Grazioli S, Gil S, An D et al. CYP61 (CCN1) overexpression induces lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2015;308:L759–65.
Kurundkar AR, Kurundkar D, Rangarajan S et al. The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016;30:2135–50.
Borkham-Kamphorst E, Schaffrath C, Van de Leur E et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-β signaling. Biochim Biophys Acta 2014;1843:902–14.
Chintala H, Krupska I, Yan L et al. The matricellular CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and Notch signaling. Development 2015;142:2364–74.
Wu J, Yan Z, Schwartz DE et al. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol 2013;190:3590–9.
Liao J, Kapadia VS, Brown LS et al. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun 2015: 6 1–12.
Moon HG, Qin Z, Quan T et al. Matrix protein CCN1 induced by bacterial DNA and CpG ODN limits lung inflammation and contributes to innate immune homeostasis. Mucosal Immunol 2015;8:243–53.
Jin Y, Kim HP, Ifedigbo E et al. Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells. Am J Respir cell Mol Biol 2005;33:297–302.
Perkowski S, Sun J, Singhal S et al. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir cell Mol Biol 2003;28:682–96.
Hummler JK, Dapaa-siakwan F, Vaidya R et al. Inhibition of Rac1 signaling down-regulates inflammasome activation and attenuates lung injury in neonatal rats exposed to hyperoxia. Neonatology 2016;111:280–8.
Lu Y, Parkyn L, Otterbein LE et al. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J Exp Med 2001;193:545–9.
Truong SV, Monick MM, Yarovinsky TO et al. Extracellular signal-regulated kinase activation delays hyperoxia-induced epithelial cell death in conditions of Akt downregulation. Am J Respir Cell Mol Biol 2004;31:611–8.
Stenmark KR, Abman SH . Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Ann Rev Physiol 2005;67:623–61.
Kunig AM, Balasubramaniam V, Markham NE et al. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–78.
Mo FE, Muntean AG, Chen CC et al. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 2002;22:8709–20.
Yan L, Chaqour B . Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of occular neovascular and fibrovascular disease therapy. J Cell Commun Signal 2013;7:253–63.
Babic AM, Kireeva ML, Kolesnikova TV et al. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 1998;95:6355–60.
Jun JI, Lau LF . The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 2010;12:676–85.
Lee SJ, Zhang M, Hu K et al. CCN1 supresses pumonary vascular smooth muscle contraction in response to hyperoxia. Pulm Circul 2015;5:716–722.
Nardiello C, Mižíková I, Morty RE . Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res 2017;367:457–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
S.W. was financially supported by Project Newborn from the University of Miami and Micah Batchelor Award from the Batchelor Foundation.
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Vaidya, R., Zambrano, R., Hummler, J. et al. Recombinant CCN1 prevents hyperoxia-induced lung injury in neonatal rats. Pediatr Res 82, 863–871 (2017). https://doi.org/10.1038/pr.2017.160
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.160
This article is cited by
-
Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway
Respiratory Research (2020)
-
Effects of Klotho supplementation on hyperoxia-induced renal injury in a rodent model of postnatal nephrogenesis
Pediatric Research (2020)
-
The matricellular protein CCN1 in tissue injury repair
Journal of Cell Communication and Signaling (2018)
-
CCN5 in alveolar epithelial proliferation and differentiation during neonatal lung oxygen injury
Journal of Cell Communication and Signaling (2018)