Abstract
Background
The intratracheal (IT) administration of budesonide using surfactant as a vehicle has been shown to reduce the incidence of bronchopulmonary dysplasia (BPD) in preterm infants. The objective of this study was to characterize the in vitro characteristics and in vivo safety and efficacy of the extemporaneous combination of budesonide and poractant alfa.
Methods
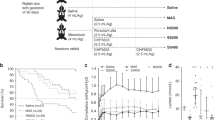
The stability, minimum surface tension, and viscosity of the preparation were evaluated by means of high-performance liquid chromatography (HPLC), Wilhelmy balance, and Rheometer, respectively. The safety and efficacy of the IT administration of the mixture were tested in two respiratory distress syndrome (RDS) animal models: twenty-seventh day gestational age premature rabbits and surfactant-depleted adult rabbits.
Results
A pre-formulation trial identified a suitable procedure to ensure the homogeneity and stability of the formulation. Wilhelmy Balance tests clarified that budesonide supplementation has no detrimental effect on poractant alfa surface tension activity. The addition of budesonide to poractant alfa did not affect the physiological response to surfactant treatment in both RDS animal models, and was associated to a significant reduction of lung inflammation in surfactant-depleted rabbits.
Conclusion
Our in vitro and in vivo analysis suggests that the IT administration of a characterized extemporaneous combination of poractant alfa and budesonide is a safe and efficacious procedure in the context of RDS.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Liggins GC, Howie RN . A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–25.
Collaborative European Multicenter Study Group. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Collaborative European Multicenter Study Group. Pediatrics 1988;82:683–91.
Guillén Ú, Weiss EM, Munson D et al. Guidelines for the management of extremely premature deliveries: a systematic review. Pediatrics 2015;136:343–50.
Hillman NH, Moss TJM, Kallapur SG et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 2007;176:575–81.
Vento M, Moro M, Escrig R et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124:e439–49.
Speer CP . Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology 2009;95:353–61.
Hayes D, Feola DJ, Murphy BS, Shook LA, Ballard HO . Pathogenesis of bronchopulmonary dysplasia. Respiration 2010;79:425–36.
Jensen EA, Schmidt B . Epidemiology of bronchopulmonary dysplasia. Birth Defects Res Part A Clin Mol Teratol 2014;100:145–57.
Bhandari A, Carroll C, Bhandari V . BPD following preterm birth: a model for chronic lung disease and a substrate for ARDS in childhood. Front Pediatr 2016;4:60.
Speer CP . Neonatal respiratory distress syndrome: an inflammatory disease? In. Neonatology 2011;99:316–9.
Bassler D, Plavka R, Shinwell ES et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med 2015;373:1497–506.
Baud O, Maury L, Lebail F et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016;387:1827–36.
Nakamura T, Yonemoto N, Nakayama M et al. Early inhaled steroid use in extremely low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2016;101:F552–6.
Yeh TF, Chen CM, Wu SY et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med 2016;193:86–95.
Yeh TF, Lin HC, Chang CH et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics 2008;121:e1310–8.
Yeh TF, Torre JA, Rastogi A, Anyebuno MA, Pildes RS . Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. J Pediatr 1990;117:273–82.
Lin YJ, Yeh TF, Hsieh WS, Chi YC, Lin HC, Lin CH . Prevention of chronic lung disease in preterm infants by early postnatal dexamethasone therapy. Pediatr Pulmonol 1999;27:21–6.
Shinwell ES, Karplus M, Reich D et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2000;83:F177–81.
Lin YJ, Lin HC,; Lin CH, Chang SJ, Yeh T. Double-blind controlled trial of endotracheal instillation of budesonide in preterm infants with RDS: a preliminary report. Pediatric Academic Society (PAS), 2000 (http://www.abstract2view.com/pasall/view.php?nu=PASOL_2436).
Mazela J, Polin RA . Aerosol delivery to ventilated newborn infants: historical challenges and new directions. Eur. J. Pediatr. 2011;170:433–44.
Ramanathan R, Bhatia JJ, Sekar K, Ernst FR . Mortality in preterm infants with respiratory distress syndrome treated with poractant alfa, calfactant or beractant: a retrospective study. J Perinatol 2013;33:119–25.
Zuo YY, Zhang H, Wang YE, Neal CR . Differential effects of cholesterol and budesonide on biophysical properties of clinical surfactant. Pediatr Res 2012;71:316–23.
Todorov R, Exerowa D, Alexandrova L et al. Behavior of thin liquid films from aqueous solutions of a pulmonary surfactant in presence of corticosteroids. Colloids Surf A Physicochem Eng Asp 2017;521:105–11.
Dani C, Corsini I, Burchielli S et al. Natural surfactant combined with beclomethasone decreases lung inflammation in the preterm lamb. Respiration 2011;82:369–76.
Dani C, Corsini I, Burchielli S et al. Natural surfactant combined with beclomethasone decreases oxidative lung injury in the preterm lamb. Pediatr Pulmonol 2014;44:1159–67.
Ricci F, Murgia X, Razzetti R, Pelizzi N, Salomone F . In vitro and in vivo comparison between poractant alfa and the new generation synthetic surfactant CHF5633. Pediatr Res 2017;81:369–75.
Ricci F, Catozzi C, Murgia X et al. Physiological, biochemical, and biophysical characterization of the lung-lavaged spontaneously-breathing rabbit as a model for respiratory distress syndrome. PLoS ONE 2017;12:e0169190.
Mrozek JP, Smith KM, Bing DR et al. Exogenous surfactant and partial liquid ventilation: physiologic and pathologic effects. Am J Respir Crit Care Med 1997;156:1058–65.
Zimmermann AM, Roberts KD, Lampland AL et al. Improved gas exchange and survival after KL-4 surfactant in newborn pigs with severe acute lung injury. Pediatr Pulmonol 2010;45:782–8.
Tam EWY, Chau V, Ferriero DM et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Transl Med 2011;3:1–6.
Zhang H, Wang YE, Neal CR, Zuo YY . Differential effects of cholesterol and budesonide on biophysical properties of clinical surfactant. Pediatr Res 2012;71:316–23.
Filoche M, Tai C-F, Grotberg JB . Three-dimensional model of surfactant replacement therapy. Proc Natl Acad Sci USA 2015;112:9287–92.
Palmer D, Schurch S, Belik J . Effect of budesonide and salbutamol on surfactant properties. J Appl Physiol 2000;89:884–90.
López-Rodríguez E, Ospina OL, Echaide M, Taeusch HW, Pérez-Gil J . Exposure to polymers reverses inhibition of pulmonary surfactant by serum, meconium, or cholesterol in the captive bubble surfactometer. Biophys J 2012;103:1451–9.
Wang YE, Zhang H, Fan Q, Neal CR, Zuo YY . Biophysical interaction between corticosteroids and natural surfactant preparation: implications for pulmonary drug delivery using surfactant as a carrier. Soft Matter 2012;8:504.
Almlén A, Stichtenoth G, Linderholm B et al. Surfactant proteins B and C are both necessary for alveolar stability at end expiration in premature rabbits with respiratory distress syndrome. J Appl Physiol 2008;104:1101–8.
Rey-Santano C, Mielgo VE, Andres L, Ruiz-del-Yerro E, Valls-i-Soler A, Murgia X . Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr Res 2013;73:639–46.
Rey-Santano C, Mielgo VE, Murgia X et al. Cerebral and lung effects of a new generation synthetic surfactant with SP-B and SP-C analogs in preterm lambs. Pediatr Pulmonol 52:929–38.
Matute-Bello G, Downey G, Moore BB et al. An official american thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am J Resp Cell Mol Biol 2011;44:725–38.
Acknowledgements
Statement of financial support
The present study was supported by Chiesi Farmaceutici.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F.R., N.M., E.S., V.D.L., E.S., M.P., S.C., G.V., M.C., F.A., F.F.S., B.P., and F.S. are employees of Chiesi Farmaceutici (Curosurf, poractant alfa, owner). X.M. served as consultants for Chiesi Farmaceutici (Curosurf, poractant alfa, owner) in this study.
Additional information
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Ricci, F., Catozzi, C., Ravanetti, F. et al. In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration. Pediatr Res 82, 1056–1063 (2017). https://doi.org/10.1038/pr.2017.171
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.171
This article is cited by
-
Time-resolved transcriptomic profiling of the developing rabbit’s lungs: impact of premature birth and implications for modelling bronchopulmonary dysplasia
Respiratory Research (2023)
-
Intratracheal budesonide mixed with surfactant to increase survival free of bronchopulmonary dysplasia in extremely preterm infants: study protocol for the international, multicenter, randomized PLUSS trial
Trials (2023)
-
Aerosol drug delivery to spontaneously-breathing preterm neonates: lessons learned
Respiratory Research (2021)
-
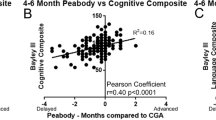
Budesonide mixed with surfactant did not affect neurodevelopmental outcomes at 6 or 18 months corrected age in observational cohorts
Journal of Perinatology (2021)
-
Surfactant lung delivery with LISA and InSurE in adult rabbits with respiratory distress
Pediatric Research (2021)