Abstract
Background
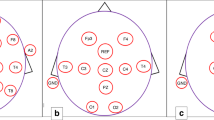
Little is known on amplitude-integrated electroencephalography (aEEG) during the first few days after birth in neonates with congenital heart disease (CHD). Our aim was, therefore, to assess electrocortical activity using aEEG within the first 72 h after birth in neonates diagnosed prenatally with CHD, and to define independent prenatal and postnatal predictors for abnormal aEEG.
Methods
Neonates with CHD who were admitted to the neonatal intensive care unit between 2010 and 2017 were retrospectively included. We assessed aEEG background patterns, sleep–wake cycling, and epileptic activity during the first 72 h after birth and defined prenatal and postnatal clinical parameters associated with aEEG patterns.
Results
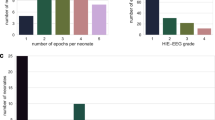
Seventy-two neonates were included. Twenty-six (36%) had mildly abnormal and six (8%) had severely abnormal aEEG background patterns at some point during the study period. Sleep–wake cycling was present in 97% of the neonates. Subclinical seizures were common (15%), whereas none of the neonates had clinical seizures. Only treatment with sedatives was a significant predictor for abnormal aEEG background patterns, explaining 56% of the variance.
Conclusion
Abnormal aEEG background patterns are common and are strongly associated with treatment with sedatives in neonates with prenatally diagnosed CHD. Future studies should assess the association between early postnatal aEEG abnormalities and neurodevelopmental outcome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Marino BS, Lipkin PH, Newburger JW et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012;126:1143–72.
Schaefer C, von Rhein M, Knirsch W et al. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol 2013;55:1143–9.
Toet MC, Lemmers PM . Brain monitoring in neonates. Early Hum Dev 2009;85:77–84.
Mulkey SB, Yap VL, Bai S et al. Amplitude-integrated EEG in newborns with critical congenital heart disease predicts preoperative brain magnetic resonance imaging findings. Pediatr Neurol 2015;52:599–605.
Ter Horst HJ, Mud M, Roofthooft MT, Bos AF . Amplitude integrated electroencephalographic activity in neonates with congenital heart disease before surgery. Early Hum Dev 2010;86:759–64.
Gunn JK, Beca J, Penny DJ et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg 2012;93:170–6.
Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS . Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med 2012;38:1539–47.
Latal B, Wohlrab G, Brotschi B, Beck I, Knirsch W, Bernet V . Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J Pediatr 2016;178:55–60.e1.
Friedman AH, Fahey JT . The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol 1993;17:106–21.
Hillman NH, Kallapur SG, Jobe AH . Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012;39:769–83.
Masoller N, Sanz-Cortes M, Crispi F et al. Severity of fetal brain abnormalities in congenital heart disease in relation to the main expected pattern of in utero brain blood supply. Fetal Diagn Ther 2016;39:269–78.
Masoller N, Martinez JM, Gomez O et al. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2014;44:182–7.
Hahn E, Szwast A, Cnota J et al. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet Gynecol 2016;47:460–5.
El-Naggar WI, Keyzers M, McNamara PJ . Role of amplitude-integrated electroencephalography in neonates with cardiovascular compromise. J Crit Care 2010;25:317–21.
de Vries LS, Toet MC . Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol 2006;33:619–32 vi.
Peyvandi S, De Santiago V, Chakkarapani E et al. Association of prenatal diagnosis of critical congenital heart disease with postnatal brain development and the risk of brain injury. JAMA Pediatr 2016;170:e154450.
Limperopoulos C, Majnemer A, Rosenblatt B et al. Association between electroencephalographic findings and neurologic status in infants with congenital heart defects. J Child Neurol 2001;16:471–6.
Naim MY, Gaynor JW, Chen J et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2015;150:169–80.
Pinchefsky EF, Hahn CD . Outcomes following electrographic seizures and electrographic status epilepticus in the pediatric and neonatal ICUs. Curr Opin Neurol 2017;30:156–64.
Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S . Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed 2008;93:F187–91.
van Laerhoven H, de Haan TR, Offringa M, Post B, van der Lee JH . Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics 2013;131:88–98.
Osredkar D, Toet MC, van Rooij LG, Van Huffelen A, Groenendaal F, De Vries LS . Sleep–wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics 2005;115:327–332.
ter Horst HJ, Sommer C, Bergman KA, Fock JM, van Weerden TW, Bos AF . Prognostic significance of amplitude-integrated EEG during the first 72 h after birth in severely asphyxiated neonates. Pediatr Res 2004;55:1026–33.
Young GB, da Silva OP . Effects of morphine on the electroencephalograms of neonates: a prospective, observational study. Clin Neurophysiol 2000;111:1955–60.
Bell AH, Greisen G, Pryds O . Comparison of the effects of phenobarbitone and morphine administration on EEG activity in preterm babies. Acta Paediatr 1993;82:35–9.
Bernet V, Latal B, Natalucci G, Doell C, Ziegler A, Wohlrab G . Effect of sedation and analgesia on postoperative amplitude-integrated EEG in newborn cardiac patients. Pediatr Res 2010;67:650–5.
Olischar M, Davidson AJ, Lee KJ, Hunt RW . Effects of morphine and midazolam on sleep-wake cycling in amplitude-integrated electroencephalography in post-surgical neonates >/= 32 weeks of gestational age. Neonatology 2012;101:293–300.
Meher S, Hernandez-Andrade E, Basheer SN, Lees C . Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: a systematic review. Ultrasound Obstet Gynecol 2015;46:398–404.
Bunt JEH, Gavilanes AWD, Reulen JPH, Blanco CE, Vles JSH . The influence of acute hypoxemia and hypovolemic hypotension of neuronal brain activity measured by the cerebral function monitor in newborn piglets. Neuropediatrics 1996;27:260–4.
Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C . Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 1999;103:402–8.
Acknowledgements
This study was part of the research program of the Graduate School of Medical Sciences, Research Institutes BCN-BRAIN and GUIDE, and University of Groningen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
M.J.M. and S.J.K. were financially supported by the Junior Scientific Master Class of the University of Groningen.
Supplementary material is linked to the version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Mebius, M., Oostdijk, N., Kuik, S. et al. Amplitude-integrated electroencephalography during the first 72 h after birth in neonates diagnosed prenatally with congenital heart disease. Pediatr Res 83, 798–803 (2018). https://doi.org/10.1038/pr.2017.311
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.311
This article is cited by
-
The impact of sleep in high-risk infants
Pediatric Research (2025)
-
Neuromonitoring practices for neonates with congenital heart disease: a scoping review
Pediatric Research (2025)
-
Spectral EEG in Congenital Heart Disease: A Case–Control Study in Infants Undergoing Cardiac Surgery
Pediatric Cardiology (2025)
-
Continuous electroencephalography (cEEG) in infants with congenital heart disease (CHD)
Pediatric Research (2023)
-
CeRebrUm and CardIac Protection with ALlopurinol in Neonates with Critical Congenital Heart Disease Requiring Cardiac Surgery with Cardiopulmonary Bypass (CRUCIAL): study protocol of a phase III, randomized, quadruple-blinded, placebo-controlled, Dutch multicenter trial
Trials (2022)