Abstract
Background
Perinatal exposure to glucocorticoids and elevated endogenous glucocorticoid levels during childhood can have detrimental effects on the developing brain. Here, we examined the impact of glucocorticoid treatment during childhood on brain volumes.
Methods
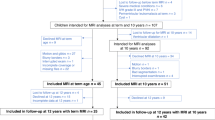
A total of 30 children and adolescents with rheumatic or nephrotic disease previously treated with glucocorticoids and 30 controls matched on age, sex, and parent education underwent magnetic resonance imaging (MRI) of the brain. Total cortical gray and white matter, brain, intracranial volume, and total cortical thickness and surface area were derived from MRI scans.
Results
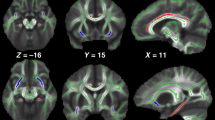
Patients had significantly smaller gray and white matter and total brain volumes relative to healthy controls. Brain volume differences disappeared when accounting for intracranial volume, as patients had relatively smaller intracranial volumes. Group differences were mainly driven by the children with rheumatic disease. Total cortical thickness and cortical surface area did not significantly differ between groups. We found no significant associations between glucocorticoid-treatment variables and volumetric measures.
Conclusion
Observed smaller total brain, cortical gray, and white matter volumes in children and adolescents previously treated with glucocorticoids compared with that in healthy controls may reflect both developmental and degenerative processes. Prospective longitudinal studies are warranted to clarify whether findings are related to treatment or disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cao-Lei L, Suwansirikul S, Jutavijittum P et al. Glucocorticoid receptor gene expression and promoter CpG modifications throughout the human brain. J Psychiatr Res 2013;47:1597–607.
Doyle LW, Ehrenkranz RA, Halliday HL . Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2014;5:CD001146.
Damsted SK, Born AP, Paulson OB, Uldall P . Exogenous glucocorticoids and adverse cerebral effects in children. Eur J Paediatr Neurol 2011;15:465–77.
Salpietro V, Polizzi A, Di RG et al. Adrenal disorders and the paediatric brain: pathophysiological considerations and clinical implications. Int J Endocrinol 2014;2014:282489.
Davis EP, Sandman CA, Buss C et al. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry 2013;74:647–55.
Malaeb SN, Stonestreet BS . Steroids and injury to the developing brain: net harm or net benefit? Clin Perinatol 2014;41:191–208.
de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M . Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998;19:269–301.
de Kloet ER . From receptor balance to rational glucocorticoid therapy. Endocrinology 2014;155:2754–69.
McEwen BS, Angulo J, Cameron H et al. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry 1992;31:177–99.
McEwen BS . Corticosteroids and hippocampal plasticity. Ann N Y Acad Sci 1994;746:134–42.
Tamnes CK, Herting MM, Goddings AL et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci 2017;37:3402–12.
Jernigan TL, Baare WF, Stiles J, Madsen KS . Postnatal brain development: structural imaging of dynamic neurodevelopmental processes. Prog Brain Res 2011;189:77–92.
Mills KL, Goddings AL, Herting MM et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage 2016;141:273–81.
Coupe P, Catheline G, Lanuza E, Manjon JV . Towards a unified analysis of brain maturation and aging across the entire lifespan: a MRI analysis. Hum Brain Mapp 2017;38:5501–18.
Giedd JN, Stockman M, Weddle C et al. Anatomic magnetic resonance imaging of the developing child and adolescent brain and effects of genetic variation. Neuropsychol Rev 2010;20:349–61.
Walhovd KB, Fjell AM, Giedd J et al. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb Cortex 2017;27:1472–81.
Lenroot RK, Gogtay N, Greenstein DK et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007;36:1065–73.
Amlien IK, Fjell AM, Tamnes CK et al. Organizing principles of human cortical development—thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy. Cereb Cortex 2016;26:257–67.
Brown TT, Kuperman JM, Chung Y et al. Neuroanatomical assessment of biological maturity. Curr Biol 2012;22:1693–8.
Paus T . Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn 2010;72:26–35.
Holm SK, Vestergaard M, Madsen KS et al. Children and adolescents previously treated with glucocorticoids display lower verbal intellectual abilities. Acta Paediatr 2015;104:784–91.
Dale AM, Fischl B, Sereno MI . Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–94.
Fischl B, Sereno MI, Dale AM . Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207.
Ghosh SS, Kakunoori S, Augustinack J et al. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage 2010;53:85–93.
Fischl B, Dale AM . Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–5.
Buckner RL, Head D, Parker J et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–38.
Mills KL, Tamnes CK . Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci 2014;9:172–90.
Scheffler C, Greil H, Hermanussen M . The association between weight, height, and head circumference reconsidered. Pediatr Res 2017;81:825–30.
Merke DP, Giedd JN, Keil MF et al. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J Clin Endocrinol Metab 2005;90:2531–6.
Patil CG, Lad SP, Katznelson L, Laws ER Jr . Brain atrophy and cognitive deficits in Cushing's disease. Neurosurg Focus 2007;23:1–4.
Ito M, Takao T, Okuno T, Mikawa H . Sequential CT studies of 24 children with infantile spasms on ACTH therapy. Dev Med Child Neurol 1983;25:475–80.
Konishi Y, Yasujima M, Kuriyama M et al. Magnetic resonance imaging in infantile spasms: effects of hormonal therapy. Epilepsia 1992;33:304–9.
Yano E . Apparent cerebral atrophic findings on cranial computed tomography in nephrotic children with steroid therapy and in patients of infantile spasms with ACTH therapy. Kurume Med J 1981;28:63–77.
Cheong JL, Burnett AC, Lee KJ et al. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J Pediatr 2014;164:737–43.
Gaspari S, Marcovecchio ML, Breda L, Chiarelli F . Growth in juvenile idiopathic arthritis: the role of inflammation. Clin Exp Rheumatol 2011;29:104–110.
Hochberg Z . Mechanisms of steroid impairment of growth. Horm Res 2002;58 (Suppl 1): 33–8.
Webb EA, O'Reilly MA, Clayden JD et al. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain 2012;135:216–27.
Aberg D . Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr Dev 2010;17:63–76.
Eddy AA, Symons JM . Nephrotic syndrome in childhood. Lancet 2003;362:629–39.
Ravelli A, Martini A . Juvenile idiopathic arthritis. Lancet 2007;369:767–78.
Acknowledgements
Our greatest acknowledgment is directed toward all participating children and their parents. Furthermore, The Danish Research Council for Independent Research, the Lundbeck Foundation, and The Ville Heise Foundation are acknowledged for kindly funding the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Financial disclosure: Hartwig Siebner has received honoraria as speaker from Lundbeck A/S, Denmark, Biogen Idec, Denmark, Genzyme, Denmark, and MerckSerono, Denmark, honoraria from Elsevier Publishers, Amsterdam, The Netherlands, and Springer Publishing, Stuttgart, Germany, and travel support from MagVenture, Denmark. The remaining authorsdeclare no conflict of interest.
Additional information
Statement of Financial Support
All phases of this study were kindly supported by The Danish Council for Independent Research (grant number: 09-071546), The Lundbeck Foundation (grant number: R48-A4968), and The Ville Heise Foundation.
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Holm, S., Madsen, K., Vestergaard, M. et al. Total brain, cortical, and white matter volumes in children previously treated with glucocorticoids. Pediatr Res 83, 804–812 (2018). https://doi.org/10.1038/pr.2017.312
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.312
This article is cited by
-
Limbic system abnormalities in episodic cluster headache: a 7T MRI multimodal study
The Journal of Headache and Pain (2025)
-
Involvement of the ipsilateral-to-the-pain anterior–superior hypothalamic subunit in chronic cluster headache
The Journal of Headache and Pain (2024)
-
Glucocorticoid treatment for non-cerebral diseases in children and adolescents is associated with differences in uncinate fasciculus microstructure
Pediatric Research (2022)