Abstract
Background
Preterm birth is associated with an increased risk of cerebellar injury. The aim of this study was to assess the impact of cerebellar hemorrhages (CBH) on cerebral white matter microstructural tissue organization and cerebellar volume at term-equivalent age (TEA) in extremely preterm infants. Furthermore, we aimed to evaluate the association between CBH and neurodevelopmental outcome in late infancy.
Methods
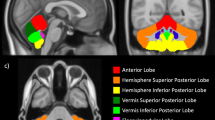
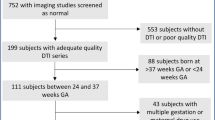
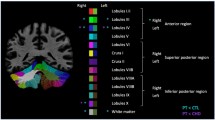
A total of 24 preterm infants with punctate CBH were included and each matched to two preterm control infants. T1-, T2-weighted images and diffusion-weighted imaging were acquired on a 3T magnetic resonance imaging (MRI) system. Regions of interest were drawn on a population-specific neonatal template and automatically registered to individual fractional anisotropy (FA) maps. Brain volumes were automatically computed. Neurodevelopmental outcome was assessed using the Bayley scales of Infant and Toddler Development at 2 years of corrected age.
Results
CBHs were not significantly related to FA in the posterior limb of the internal capsule and corpus callosum or to cerebellar volume. Infants with CBH did not have poorer neurodevelopmental outcome compared with control infants.
Conclusion
These findings suggest that the impact of mild CBH on early macroscale brain development may be limited. Future studies are needed to assess the effects of CBH on long-term neurodevelopment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brossard-Racine M, du Plessis AJ, Limperopoulos C . Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum 2015;14:151–64.
Volpe JJ . Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 2009;24:1085–104.
Steggerda SJ, De Bruine FT, van den Berg-Huysmans AA et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum 2013;12:794–801.
Limperopoulos C, Benson CB, Bassan H et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 2005;116:717–24.
Dyet LE, Kennea N, Counsell SJ et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 2006;118:536–48.
Bednarek N, Akhavi A, Pietrement C, Mesmin F, Loron G, Morville P . Outcome of cerebellar injury in very low birth-weight infants: 6 case reports. J Child Neurol 2008;23:906–11.
Limperopoulos C, Bassan H, Gauvreau K et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120:584–93.
Tam EW, Rosenbluth G, Rogers EE et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr 2011;158:245–50.
Monakow CV, Monakow CV . Brain and behavior I: mood, states and mind. In: Pibram KH, ed. Diaschisis. Penguin Books, Baltimore 1969:27–36.
Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ . Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex 2014;24:728–36.
Limperopoulos C, Soul JS, Haidar H et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics 2005;116:844–50.
Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ . Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res 2010;68:145–50.
Bolduc ME, Du Plessis AJ, Evans A et al. Cerebellar malformations alter regional cerebral development. Dev Med Child Neurol 2011;53:1128–34.
Huppi PS, Dubois J . Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 2006;11:489–97.
Thompson DK, Inder TE, Faggian N et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 2012;59:3571–81.
Rose J, Cahill-Rowley K, Vassar R et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18-22 mo of age: an MRI and DTI study. Pediatr Res 2015;78:700–8.
van Kooij BJ, de Vries LS, Ball G et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol 2012;33:188–94.
Kidokoro H, Neil JJ, Inder TE . New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–14.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE . Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94.
Leemans A, Jeurissen B, Sijbers J, Jones DK. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proceedings of the 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine, Honolulu, USA, 2009, 3537.
Leemans A, Jones DK . The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61:1336–49.
Tax CM, Otte WM, Viergever MA, Dijkhuizen RM, Leemans A . REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn Reson Med 2015;73:794–808.
Reuter M, Schmansky NJ, Rosas HD, Fischl B 2012 Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61:1402–18.
Anbeek P, Isgum I, van Kooij BJ et al. Automatic segmentation of eight tissue classes in neonatal brain MRI. PLoS ONE 2013;8:e81895.
Bayley N. Bayley Scales of Infant and Toddler Development-Third Edition. Harcourt Assessment NCS. San Antonio, TX: Pearson, 2006.
Ball G, Pazderova L, Chew A et al. Thalamocortical connectivity predicts cognition in children born preterm. Cereb Cortex 2015;25:4310–8.
Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C . Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995;37:885–93.
Dailey AT, McKhann GM 2nd, Berger MS . The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg 1995;83:467–75.
Sagiuchi T, Ishii K, Aoki Y et al. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med 2001;15:157–60.
Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G . Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 2009;252:190–9.
Messerschmidt A, Prayer D, Brugger PC et al. Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol 2008;12:455–60.
Castelli F, Frith C, Happe F, Frith U . Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839–49.
Holdefer RN, Miller LE, Chen LL, Houk JC . Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol 2000;84:585–90.
Schmahmann JD . Cognition and the cerebellum. Neurology 2004;63:1991.
Ochiai M, Ichiyama M, Iwayama M, Sakai Y, Yoshida K, Hara T . Longitudinal study of very low birth weight infants until 9years of age; attention deficit hyperactivity and autistic features are correlated with their cognitive functions. Early Hum Dev 2015;91:783–6.
Fushimi Y, Miki Y, Okada T et al. Fractional anisotropy and mean diffusivity: comparison between 3.0- T and 1.5- T diffusion tensor imaging with parallel imaging using histogram and region of interest analysis. NMR Biomed 2007;20:743–8.
Altman DG, Bland JM . Absence of evidence is not evidence of absence. Br Med J 1995;311:485.
Pieterman K, Batalle D, Dudink J et al. Cerebello-cerebral connectivity in the developing brain. Brain Struct Funct 2016;222:1625–34.
Xu G, Takahashi E, Folkerth RD et al. Radial coherence of diffusion tractography in the cerebral white matter of the human fetus: neuroanatomic insights. Cereb Cortex 2014;24:579–92.
Smith SM, Jenkinson M, Johansen-Berg H et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505.
Acknowledgements
We thank Nathalie H.P. Claessens and Karina J. Kersbergen for their contribution toward the data collection. We would also like to thank Ingrid C. van Haastert, for her careful evaluation at the outpatient clinic of the children included in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Statement of Financial Support
R.S. was awarded a Van Walree Scholarship of the Royal Academy of Arts and Sciences (Koninklijke Nederlandse Academie van Wetenschappen) to present results of this study at the Pediatric Academic Societies Meeting in San Diego in 2015. K.K. is supported by a grant from the Wilhelmina Children’s Hospital Research Fund (Vrienden WKZ) to Martijn P. van den Heuvel. The research of A.L. is supported by VIDI Grant 639.072.411 from the Netherlands Organization for Scientific Research (NWO).
Supplementary material is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Senden, R., Keunen, K., van der Aa, N. et al. Mild cerebellar injury does not significantly affect cerebral white matter microstructural organization and neurodevelopmental outcome in a contemporary cohort of preterm infants. Pediatr Res 83, 1004–1010 (2018). https://doi.org/10.1038/pr.2018.10
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2018.10
This article is cited by
-
MRI at term equivalent in preterm infants: the wise choice
Pediatric Research (2018)