Abstract
Background
Postnatally, the immature left ventricle (LV) is subjected to high systemic afterload. Hypothesizing that LV pumping would change during transition–adaptation, we analyzed the LV in preterm infants (GA≤32+6), clinically stable or with a hemodynamically significant patent ductus arteriosus (hPDA) by applying a pump model.
Methods
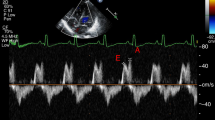
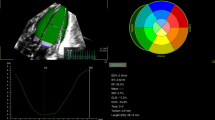
Pumping was characterized by EA (effective arterial elastance, reflecting afterload), EES (end-systolic LV elastance, reflecting contractility), EA/EES coupling ratios, descriptive EA:EES relations, and EA/EES graphs. Data calculated from echocardiography and blood pressure were analyzed by diagnosis (S group: clinically stable, no hPDA, n=122; hPDA group, n=53) and by periods (early transition: days of life 1–3; late transition: 4–7; and adaptation: 8–30).
Results
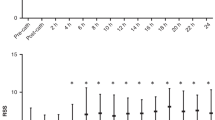
S group: LV pumping was characterized by an increased EA/EES coupling ratio of 0.65 secondary to low EES in early transition, a tandem rise of both EA and EES in late transition, and an EA/EES coupling ratio of 0.45 secondary to high EES in adaptation; hPDA group: time-trend analyses showed significantly lower EA (P<0.0001) and EES (P=0.006). Therefore, LV pumping was characterized by a lower EA/EES coupling ratio (P=0.088) throughout transition–adaptation.
Conclusions
In stable infants, facing high afterload, the immature LV, enhanced by the physiological PDA, increases its contractility. In hPDA, facing low afterload, the overloaded immature LV exhibits a consistently lower contractility.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Azhibekov T, Soleymani S, Lee BH et al. Hemodynamic monitoring of the critically ill neonate: an eye on the future. Semin Fetal Neonatal Med 2015;20:246–254.
Engle WD . Blood pressure in the very low birth weight neonate. Early Hum Dev 2001;62:97–130.
Noori S, Seri I . Pathophysiology of newborn hypotension outside the transitional period. Early Hum Dev 2005;81:399–404.
El Hajjar M, Vaksmann G, Rakza T et al. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed 2005;90:F419–F422.
Jain A, Shah PS . Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr 2015;169:863–872.
Evans N, Kluckow M . Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 1996;75:F183–F186.
Finnemore A, Groves A . Physiology of the fetal and transitional circulation. Semin Fetal Neonatal Med 2015;20:210–216.
Hamdani N, Herwig M, Linke WA . Tampering with springs: phosphorylation of titin affecting the mechanical function of cardiomyocytes. Biophys Rev 2017;9:225–237.
Walker JS, de Tombe PP . Titin and the developing heart. Circ Res 2004;94:860–862.
Prsa M, Sun L, van Amerom J et al. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging 2014;7:663–670.
Fernandez Pineda L, Tamariz-Martel Moreno A, Maitre Azcarate MJ et al. Contribution of Doppler atrioventricular flow waves to ventricular filling in the human fetus. Pediatr Cardiol 2000;21:422–428.
Bhorat I, Bagratee J, Reddy T . Gestational age-adjusted trends and reference intervals of the Modified Myocardial Performance Index (Mod-MPI) and its components, with its interpretation in the context of established cardiac physiological principles. Prenat Diagn 2014;34:1031–1036.
Tonino P, Kiss B, Strom J et al. The giant protein titin regulates the length of the striated muscle thick filament. Nat Commun 2017;8:1041–1049.
Sunagawa K, Maughan WL, Burkhoff D et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773–H780.
Starling MR . Left ventricular–arterial coupling relations in the normal human heart. Am Heart J 1993;125:1659–1666.
Borlaug BA, Kass DA . Ventricular–vascular interaction in heart failure. Heart Fail Clin 2008;4:23–36.
Chantler PD, Lakatta EG . Arterial–ventricular coupling with aging and disease. Front Physiol 2012;3:90.
Chantler PD, Lakatta EG, Najjar SS . Arterial–ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 2008;105:1342–1351.
Tanoue Y, Sese A, Ueno Y et al. Bidirectional Glenn procedure improves the mechanical efficiency of a total cavopulmonary connection in high-risk fontan candidates. Circulation 2001;103:2176–2180.
Chowdhury SM, Butts RJ, Taylor CL et al. Validation of noninvasive measures of left ventricular mechanics in children: a simultaneous echocardiographic and conductance catheterization study. J Am Soc Echocardiogr 2016;29:640–647.
Chen CH, Fetics B, Nevo E et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 2001;38:2028–2034.
Kass DA . Age-related changes in venticular–arterial coupling: pathophysiologic implications. Heart Fail Rev 2002;7:51–62.
Taborsky HS . Arterial–Ventricular Coupling in Term Neonates and Infants up to 6 months, A Retrospective Data Analysis.. Vienna: Pediatric and Adolescence Medicine. Medical University of Vienna: Vienna, 2015: 69.
Engel J, Baumgartner S, Novak S et al. Ventriculo-arterial coupling in children with Still’s murmur. Physiol Rep 2014: 2.
de Boode WP, Singh Y, Gupta S et al. Recommendations for neonatologist performed echocardiography in Europe: Consensus Statement endorsed by European Society for Paediatric Research (ESPR) and European Society for Neonatology (ESN). Pediatr Res 2016;80:465–471.
Teichholz LE, Kreulen T, Herman MV et al. Problems in echocardiographic volume determinations: echocardiographic–angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976;37:7–11.
Kelly RP, Ting CT, Yang TM et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86:513–521.
de Waal K, Phad N, Collins N et al. Cardiac remodeling in preterm infants with prolonged exposure to a patent ductus arteriosus. Congenit Heart Dis 2017;12:364–372.
Ky B, French B, May Khan A et al. Ventricular–arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol 2013;62:1165–1172.
Burkhoff D . Pressure–volume loops in clinical research: a contemporary view. J Am Coll Cardiol 2013;62:1173–1176.
Meyer S, Sander J, Graber S et al. Agreement of invasive versus non-invasive blood pressure in preterm neonates is not dependent on birth weight or gestational age. J Paediatr Child Health 2010;46:249–254.
Nagasawa H, Kohno Y, Yamamoto Y et al. Methodologic comparison of left ventricular stroke volumes in the early neonatal period by echocardiography. Pediatr Cardiol 2014;35:1415–1420.
Burkhoff D, Sagawa K . Ventricular efficiency predicted by an analytical model. Am J Physiol 1986;250:R1021–R1027.
Khosroshahi HE, Ozkan EA, Kilic M . Arterial and left ventricular end-systolic elastance in normal children. Eur Rev Med Pharmacol Sci 2014;18:3260–3266.
Pladys P, Wodey E, Beuchee A et al. Left ventricle output and mean arterial blood pressure in preterm infants during the 1st day of life. Eur J Pediatr 1999;158:817–824.
Kluckow M, Evans N . Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 2000;82:F182–F187.
Antonini-Canterin F, Carerj S, Di Bello V et al. Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist’s point of view. Eur J Echocardiogr 2009;10:36–43.
Schwarz CE, Preusche A, Baden W et al. Repeatability of echocardiographic parameters to evaluate the hemodynamic relevance of patent ductus arteriosus in preterm infants: a prospective observational study. BMC Pediatr 2016;16:18.
Noori S, McNamara P, Jain A et al. Catecholamine-resistant hypotension and myocardial performance following patent ductus arteriosus ligation. J Perinatol 2015;35:123–127.
El-Khuffash A, Weisz DE, McNamara PJ . Reflections of the changes in patent ductus arteriosus management during the last 10 years. Arch Dis Child Fetal Neonatal Ed 2016;101:F474–F478.
Nagata H, Ihara K, Yamamura K et al. Left ventricular efficiency after ligation of patent ductus arteriosus for premature infants. J Thorac Cardiovasc Surg 2013;146:1353–1358.
Noori SSI . The very low birth weight neonate with a hemodynamically significant ductus arteriosus during the first postnatal week In: Polin R eds. Hemodynamics and Cardiology. Neonatology Questions and Controversies 2nd edn. New York: Columbia University Medical Center, 2008: 269–291.
Acknowledgements
We thank Dave Hill for his valuable assistance in preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Baumgartner, S., Olischar, M., Wald, M. et al. Left ventricular pumping during the transition–adaptation sequence in preterm infants: impact of the patent ductus arteriosus. Pediatr Res 83, 1016–1023 (2018). https://doi.org/10.1038/pr.2018.22
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2018.22
This article is cited by
-
Impact of a patent ductus arteriosus on non-invasive pre- and post-ductal blood pressures in extremely preterm infants during the first two postnatal weeks
Journal of Perinatology (2025)
-
Clinical determinants of cerebrovascular reactivity in very preterm infants during the transitional period
Pediatric Research (2022)