Abstract
Background
Twin studies suggest that genetic factors may account for up to 50% increased risk for necrotizing enterocolitis (NEC), but genome-wide association studies for NEC are lacking.
Methods
Genotyping was done on Illumina BeadChip, followed by analysis using PLINK with logistic regression under an additive model.
Results
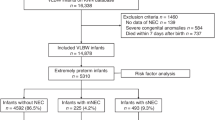
Among 751 extremely-low-birth-weight (<1,000 g, >401 g) neonates, 30 had surgical NEC. Two hundred and sixty-one single-nucleotide polymorphisms (SNPs) showed association with NEC at P<0.05, of which 35 were significant at P<10−7. Minor allele(s) in a cluster of SNPs spanning a 43-kb region of chromosome 8 (8q23.3) conferred an odds ratio of 4.72 (95% confidence interval (CI): 2.51–8.88) for elevated risk of NEC. Two smaller clusters on chromosome 14 and chromosome 11 exhibited P values of 10–7–10–8. The chromosome 8 cluster is in an intergenic region between CUB and Sushi multiple domains 3 (−1.43 Mb) and trichorhinophalangeal syndrome I (+542 kb). RNA sequencing in this region identified a potential novel open-reading frame corresponding to a long interspersed element-1 retrotransposable element.
Conclusion
Genetic variation in an intergenic region of chromosome 8 is associated with increased risk for NEC with a mechanism that is yet to be identified.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stoll BJ, Hansen NI, Bell EF et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–456.
Blakely ML, Lally KP, McDonald S et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 2005;241:984–989 discussion 989–994.
Shah TA, Meinzen-Derr J, Gratton T et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol 2012;32:552–558.
Bhandari V, Bizzarro MJ, Shetty A et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 2006;117:1901–1906.
Moonen RM, Paulussen AD, Souren NY, Kessels AG, Rubio-Gozalbo ME, Villamor E . Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res 2007;62:188–190.
Bokodi G, Derzbach L, Banyasz I, Tulassay T, Vasarhelyi B . Association of interferon gamma T+874A and interleukin 12 p40 promoter CTCTAA/GC polymorphism with the need for respiratory support and perinatal complications in low birthweight neonates. Arch Dis Child Fetal Neonatal Ed 2007;92:F25–F29.
Banyasz I, Bokodi G, Vasarhelyi B et al. Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur Cytokine Netw 2006;17:266–270.
Sampath V, Le M, Lane L et al. The NFKB1 (g.−24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res 2011;169:e51–e57.
Szebeni B, Szekeres R, Rusai K et al. Genetic polymorphisms of CD14, toll-like receptor 4, and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J Pediatr Gastroenterol Nutr 2006;42:27–31.
Sankararaman S, Yanamandra K, Napper D, Caldito G, Dhanireddy R . The prevalence of platelet activating factor acetylhydrolase single nucleotide polymorphisms in relationship to necrotizing enterocolitis in Northwest Louisiana infants. Springerplus 2013;2:294.
Prencipe G, Auriti C, Inglese R, Gallusi G, Dotta A, De Benedetti F . The macrophage migration inhibitory factor −173G/C polymorphism is not significantly associated with necrotizing enterocolitis in preterm infants. J Pediatr Surg 2013;48:1499–1502.
Koroglu OA, Onay H, Erdemir G et al. Mannose-binding lectin gene polymorphism and early neonatal outcome in preterm infants. Neonatology 2010;98:305–312.
Spiegler J, Gilhaus A, Konig IR et al. Polymorphisms in the renin–angiotensin system and outcome of very-low-birthweight infants. Neonatology 2010;97:10–14.
Henderson G, Craig S, Baier RJ, Helps N, Brocklehurst P, McGuire W . Cytokine gene polymorphisms in preterm infants with necrotising enterocolitis: genetic association study. Arch Dis Child Fetal Neonatal Ed 2009;94:F124–F128.
Genomes Project C Genomes Project C Abecasis GR Genomes Project C Auton A et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56–65.
Visscher PM, Wray NR, Zhang Q et al. 10 Years of GWAS discovery: biology, function, and translation. Am J Hum Genet 2017;101:5–22.
Carlon DB, Budd AF, Lippe C, Andrew RL . The quantitative genetics of incipient speciation: heritability and genetic correlations of skeletal traits in populations of diverging Favia fragum ecomorphs. Evolution 2011;65:3428–3447.
Bell MJ, Ternberg JL, Feigin RD et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7.
Walsh MC, Kliegman RM . Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am 1986;33:179–201.
Gogarten SM, Bhangale T, Conomos MP et al. GWASTools: an R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics 2012;28:3329–3331.
Hancock DB, Soler Artigas M, Gharib SA et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet 2012;8:e1003098.
Purcell S, Neale B, Todd-Brown K et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575.
Ment LR, Aden U, Lin A et al. Gene–environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr Res 2014;75:241–250.
Mootha VK, Lepage P, Miller K et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci USA 2003;100:605–610.
Langmead B, Salzberg SL . Fast gapped-read alignment with Bowtie 2. Nat Methods 2012;9:357–359.
Trapnell C, Pachter L, Salzberg SL . TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25:1105–1111.
Huang X, Madan A . CAP3: a DNA sequence assembly program. Genome Res 1999;9:868–877.
Yee WH, Soraisham AS, Shah VS et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129:e298–e304.
Attridge JT, Clark R, Walker MW, Gordon PV . New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol 2006;26:185–188.
Ment LR, Aden U, Bauer CR et al. Genes and environment in neonatal intraventricular hemorrhage. Semin Perinatol 2015;39:592–603.
Maston GA, Evans SK, Green MR . Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet 2006;7:29–59.
Srinivasan L, Page G, Kirpalani H et al. Genome-wide association study of sepsis in extremely premature infants. Arch Dis Child Fetal Neonatal Ed 2017;102:F439–F445.
Ambalavanan N, Cotten CM, Page GP et al. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr 2015;166:531–537 and 513.
Bannert N, Kurth R . Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci USA 2004;101 (Suppl 2): 14572–14579.
Otsubo T, Okamura T, Hagiwara T, Ishizaka Y, Dohi T, Kawamura YI . Retrotransposition of long interspersed nucleotide element-1 is associated with colitis but not tumors in a murine colitic cancer model. PLoS ONE 2015;10:e0116072.
Choi LJ, Jenikova G, Hanson E et al. Coordinate down-regulation of adenylyl cyclase isoforms and the stimulatory G protein (G(s)) in intestinal epithelial cell differentiation. J Biol Chem 2010;285:12504–12511.
Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL . Regulation of plasma membrane recycling by CFTR [see comments]. Science 1992;256:530–532.
Jilling T, Kirk KL . Cyclic AMP and chloride-dependent regulation of the apical constitutive secretory pathway in colonic epithelial cells. J Biol Chem 1996;271:4381–4387.
Mandel KG, Dharmsathaphorn K, McRoberts JA . Characterization of a cyclic AMP-activated Cl-transport pathway in the apical membrane of a human colonic epithelial cell line. J Biol Chem 1986;261:704–712.
Meir M, Flemming S, Burkard N et al. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol 2015;309:G613–G624.
Moses T, Wagner L, Fleming SD . TLR4-mediated Cox-2 expression increases intestinal ischemia/reperfusion-induced damage. J Leukoc Biol 2009;86:971–980.
Jilling T, Simon D, Lu J et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 2006;177:3273–3282.
Leaphart CL, Cavallo J, Gribar SC et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol (Baltimore, Md 2007;179:4808–4820.
Devireddy LR, Green MR . Transcriptional program of apoptosis induction following interleukin 2 deprivation: identification of RC3, a calcium/calmodulin binding protein, as a novel proapoptotic factor. Mol Cell Biol 2003;23:4532–4541.
Acknowledgements
We are indebted to our medical and nursing colleagues, and the infants and their parents who agreed to take part in this study. Data collected at participating NRN sites were transmitted to RTI International, the data-coordinating center (DCC) for the NRN, which stored, managed, and analyzed the data for this study. On behalf of the network, Abhik Das (DCC PI) and Grier Page (DCC statistician) had full access to all the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors have no financial relationships relevant to this article to disclose, and the study sponsors have not been involved in any of the following: (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
Additional information
STATEMENT OF FINANCIAL SUPPORT:
The National Institutes of Health (General Clinical Research Center Grants M01 RR30, M01 RR32, M01 RR39, M01 RR70, M01 RR80, M01 RR633, M01 RR750, M01 RR997, M01 RR6022, M01 RR7122, M01 RR8084, M01 RR16587, and UL1 RR24979) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants U01 HD36790, U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD21415, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27871, U10 HD27880, U10 HD27881, U10 HD27904, U10 HD34216, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40689, and U10 HD53109) provided grant support for the Neonatal Research Network’s Genomics Study.
The funding agencies provided overall oversight for study conduct, but all data analyses and interpretation were independent of the funding agencies. Although NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Members of the Genomics Subcommittee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICD) Neonatal Research Network
C. Michael Cotten6; Grier Page7; Waldemar A. Carlo8; Edward F. Bell9; Ronald N. Goldberg6; Kurt Schibler10; Rosemary D. Higgins11; Beena G. Sood12; David K. Stevenson13; Barbara J. Stoll14; Krisa P. Van Meurs12; Karen J. Johnson9; Abhik Das15; Scott A. McDonald7; Kristin M. Zaterka-Baxter7; Kathleen A. Kennedy16; Pablo J. Sánchez17; Shahnaz Duara18; Michele C. Walsh19; Seetha Shankaran20; Sheldon B. Korones21; Neil N. Finer22; effrey C. Murray9 for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
6Department of Pediatrics, Duke University, Durham, NC; 7Social, Statistical, and Environmental Sciences, RTI International, Research Triangle Park, NC; 8Department of Pediatrics, University of Alabama at Birmingham, Birmingham, AL; 9University of Iowa, Department of Pediatrics, Iowa City, IA; 10Department of Pediatrics, University of Cincinnati, Cincinnati, OH; 11Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD; 12Department of Pediatrics, Wayne State University, Detroit, MI; 13Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University School of Medicine and Lucile Packard Children’s Hospital, Palo Alto, CA; 14Emory University School of Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta, Atlanta, GA; 15Social, Statistical, and Environmental Sciences, RTI International, Rockville, MD; 16Department of Pediatrics, University of Texas Medical School at Houston, Houston, TX; 17Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX; 18University of Miami Miller School of Medicine, Miami, FL; 19Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland, OH; 20Department of Pediatrics, Wayne State University, Detroit, MI; 21Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN; 22University of California at San Diego, San Diego, CA.
The following investigators participated in this study:
NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati.
Case Western Reserve University, Rainbow Babies and Children’s Hospital (U10 HD21364, M01 RR80)—Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Michele C. Walsh, MD MS; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, and University Hospital (U10 HD27853, M01 RR8084)—Kurt Schibler, MD; Edward F. Donovan, MD; Vivek Narendran, MD MRCP; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN.
Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (M01 RR30, U10 HD40492)—Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Kathy J. Auten, MSHS.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, M01 RR39)—Barbara J. Stoll, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development—Rosemary D. Higgins, MD; Linda L. Wright, MD; Sumner J. Yaffe, MD; Elizabeth M. McClure, Med; Stephanie Wilson Archer, MA.
RTI International (U10 HD36790)—Abhik Das, PhD; W. Kenneth Poole, PhD; Grier Page, PhD; Scott A. McDonald, BS; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70)—Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32)—Waldemar A. Carlo MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California—San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461)—Neil N. Finer, MD; Maynard R. Rasmussen, MD; David Kaegi, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Wade Rich, BSHS RRT.
University of Iowa Children’s Hospital and Mercy Medical Center (U10 HD53109, M01 RR59, UL1 TR442)—Edward F. Bell, MD; Jeffrey C. Murray, MD; Karen J. Johnson, RN.
University of Miami Holtz Children’s Hospital (U10 HD21397, M01 RR16587)—Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN.
University of Tennessee (U10 HD21415)—Sheldon B. Korones, MD; Henrietta S. Bada, MD; Tina Hudson, RN BSN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health and Hospital System, and Children’s Medical Center of Dallas (U10 HD40689, M01 RR633)—Pablo J. Sánchez, MD; Abbot R. Laptook, MD; Walid A. Salhab, MD; Susie Madison, RN; Nancy A. Miller, RN; Gaynelle Hensley, RN; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon B. Johnson General Hospital (U10 HD21373)—Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia I. Franco, RNC MSN; Anna E. Lis, RN BSN; Georgia E. McDavid, RN; Patti Pierce Tate, RCP.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385)—Seetha Shankaran, MD; Beena G. Sood, MD MS; G. Ganesh Konduri, MD; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN.
Supplementary material is linked to the online version of the paper at
Rights and permissions
About this article
Cite this article
Jilling, T., Ambalavanan, N., Cotten, C. et al. Surgical necrotizing enterocolitis in extremely premature neonates is associated with genetic variations in an intergenic region of chromosome 8. Pediatr Res 83, 943–953 (2018). https://doi.org/10.1038/pr.2018.33
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2018.33
This article is cited by
-
Therapeutic potential of human breast milk-derived exosomes in necrotizing enterocolitis
Molecular Medicine (2024)
-
Spatial multiomic landscape of the human placenta at molecular resolution
Nature Medicine (2024)
-
Comparison and Investigation of Exosomes from Human Amniotic Fluid Stem Cells and Human Breast Milk in Alleviating Neonatal Necrotizing Enterocolitis
Stem Cell Reviews and Reports (2023)
-
Advances in our understanding of the molecular pathogenesis of necrotizing enterocolitis
BMC Pediatrics (2022)
-
Hope on the horizon: promising novel therapies for necrotizing enterocolitis
Pediatric Research (2020)