Abstract
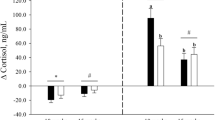
This study tested the hypothesis that the sexually dimorphic adrenocortical response to stress is already established before birth. Chronically instrumented late gestation pregnant sheep carrying 16 male and 15 female age-matched singleton fetuses were subjected to an acute episode of hypoxic stress. Maternal and fetal blood gases, adrenocorticotrophic hormone (ACTH), and cortisol were measured. In addition, six male and six female fetuses received the ACTH analog, Synacthen, and plasma cortisol was measured. During hypoxic stress, the increment in plasma cortisol was 2-fold greater in male versus females fetuses (30.6 ± 3.2 versus 14.3 ± 2.0 ng/mL; p < 0.001) mediated, in part, by greater adrenocortical sensitivity to ACTH. The data support the hypothesis tested and show that sex-specific differences in the cortisol stress response are present before birth with the output of cortisol being much greater in male than in female fetuses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HPA:
-
hypothalamic-pituitary-adrenal
- pHa:
-
arterial pH
- Paco2:
-
arterial partial pressure of carbon dioxide
- Pao2:
-
arterial partial pressure of oxygen
References
Chrousos GP 2009 Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381
Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, Funder JW 2001 GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol 173: 193–202
Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M 1993 Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14: 303–347
Kitay JI 1961 Sex differences in adrenal cortical secretion in the rat. Endocrinology 68: 818–824
Rivier C 1999 Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav 64: 739–751
Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR 2005 Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab 90: 4224–4231
Kirschbaum C, Wust S, Hellhammer D 1992 Consistent sex differences in cortisol responses to psychological stress. Psychosom Med 54: 648–657
Handa RJ, Burgess LH, Kerr JE, O'Keefe JA 1994 Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28: 464–476
McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM 1998 Neonatal sex hormones have ‘organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res 105: 295–307
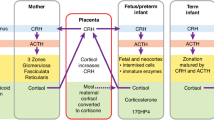
Fowden AL, Li J, Forhead AJ 1998 Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance?. Proc Nutr Soc 57: 113–122
Dodic M, May CN, Wintour EM, Coghlan JP 1998 An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155
Nyirenda MJ, Welberg LA, Seckl JR 2001 Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence?. J Endocrinol 170: 653–660
McCormick CM, Smythe JW, Sharma S, Meaney MJ 1995 Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 84: 55–61
Levine S 1994 The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci 746: 275–288
Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG 2006 Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 572: 31–44
Turner AI, Hosking BJ, Parr RA, Tilbrook AJ 2006 A sex difference in the cortisol response to tail docking and ACTH develops between 1 and 8 weeks of age in lambs. J Endocrinol 188: 443–449
Gardner DS, Van Bon BW, Dandrea J, Goddard PJ, May SF, Wilson V, Stephenson T, Symonds ME 2006 Effect of periconceptional undernutrition and gender on hypothalamic-pituitary-adrenal axis function in young adult sheep. J Endocrinol 190: 203–212
Andrew R, Phillips DI, Walker BR 1998 Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 83: 1806–1809
Lindquist TL, Beilin LJ, Knuiman MW 1997 Influence of lifestyle, coping, and job stress on blood pressure in men and women. Hypertension 29: 1–7
Fukuda S, Morimoto K 2001 Lifestyle, stress and cortisol response: review II. Environ Health Prev Med 6: 15–21
Jones CT 1977 The development of some metabolic responses to hypoxia in the foetal sheep. J Physiol 265: 743–762
Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA 1993 Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461: 431–449
Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JA, Hanson MA 1994 Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. J Physiol 477: 75–80
Gardner DS, Fowden AL, Giussani DA 2002 Adverse intrauterine conditions diminish the fetal defense against acute hypoxia by increasing nitric oxide activity. Circulation 106: 2278–2283
Giussani DA, Gardner DS, Cox DT, Fletcher AJ 2001 Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 280: R678–R685
Gardner DS, Jamall E, Fletcher AJ, Fowden AL, Giussani DA 2004 Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses. J Physiol 557: 1021–1032
Fowden AL, Mijovic J, Silver M 1993 The effects of cortisol on hepatic and renal gluconeogenic enzyme activities in the sheep fetus during late gestation. J Endocrinol 137: 213–222
Dawes GS 1968 Foetal blood-gas homeostasis during development. Proc R Soc Med 61: 1227–1231
Rudolph AM, Itskovitz J, Iwamoto H, Reuss ML, Heymann MA 1981 Fetal cardiovascular responses to stress. Semin Perinatol 5: 109–121
Lee ST, Hon EH 1963 Fetal hemodynamic response to umbilical cord compression. Obstet Gynecol 22: 553–562
Moore LG, Niermeyer S, Zamudio S 1998 Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol Suppl 27: 25–64
Ratcliffe FM, Evans JM 1993 Neonatal wellbeing after elective caesarean delivery with general, spinal, and epidural anaesthesia. Eur J Anaesthesiol 10: 175–181
Giussani DA, Gardner DS 2004 Intrauterine hypoxaemia and cardiovascular development. In: Langley-Evans SC (ed) Fetal Nutrition and Adult Disease: Programming of Chronic Disease through Fetal Exposure to Undernutrition. CAB International, Wallingford, CT, pp 55–87
Alexander DP, Forsling ML, Martin MJ, Nixon DA, Ratcliffe JG, Redstone D, Tunbridge D 1972 The effect of maternal hypoxia on fetal pituitary hormone release in the sheep. Biol Neonate 21: 219–228
Keller-Wood M, Wood CE 1991 Does the ovine placenta secrete ACTH under normoxic or hypoxic conditions?. Am J Physiol 260: R389–R395
Gardner DS, Fletcher AJ, Fowden AL, Giussani DA 2001 Plasma adrenocorticotropin and cortisol concentrations during acute hypoxemia after a reversible period of adverse intrauterine conditions in the ovine fetus during late gestation. Endocrinology 142: 589–598
Gardner DS, Fletcher AJ, Bloomfield M, Fowden AL, Giussani DA 2002 The effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol 540: 351–366
Edwards AV, Jones CT 1987 The effect of splanchnic nerve stimulation on adrenocortical activity in conscious calves. J Physiol 382: 385–396
Edwards AV, Jones CT 1987 The effect of splanchnic nerve section on the sensitivity of the adrenal cortex to adrenocorticotrophin in the calf. J Physiol 390: 23–31
Comline RS, Silver M 1961 The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol 156: 424–444
Engeland WC, Wotus C, Rose JC 1998 Ontogeny of innervation of rat and ovine fetal adrenals. Endocr Res 24: 889–898
Myers DA, Robertshaw D, Nathanielsz PW 1990 Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology 127: 2328–2335
Bloom SR, Edwards AV, Jones CT 1987 Adrenal cortical responses to vasoactive intestinal peptide in conscious hypophysectomized calves. J Physiol 391: 441–450
Jones CT, Ritchie JW 1976 Endocrine and metabolic changes associated with periods of spontaneous hypoxia in fetal sheep. Biol Neonate 29: 286–293
Jones CT, Ritchie JW, Walker D 1983 The effects of hypoxia on glucose turnover in the fetal sheep. J Dev Physiol 5: 223–235
Jones CT, Ashton IK 1976 The appearance, properties, and functions of gluconeogenic enzymes in the liver and kidney of the guinea pig during fetal and early neonatal development. Arch Biochem Biophys 174: 506–522
Jones CT, Robinson RO 1975 Plasma catecholamines in foetal and adult sheep. J Physiol 248: 15–33
Fletcher AJ, Edwards CMB, Gardner DS, Fowden AL, Giussani DA 2000 Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology 141: 3976–3982
Apatu RS, Barnes RJ 1991 Release of glucose from the liver of fetal and postnatal sheep by portal vein infusion of catecholamines or glucagon. J Physiol 436: 449–468
Whitworth JA, Williamson PM, Mangos G, Kelly JJ 2005 Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1: 291–299
Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH 1991 Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. N Engl J Med 325: 756–762
Acknowledgements
We thank Professor A.L. Fowden for her continued support with our studies, Mr. Paul Hughes and Mr. Scott Gentle for their help during surgery, Mrs. Sue Nicholls and Miss Victoria Johnson for the routine care of the animals used in this study, and Mr. Malcolm Bloomfield for the radioimmunoassays.
This article is dedicated to Dr. Andrew J.W. Fletcher. Andy was a promising young doctor and an excellent scientist, who died suddenly, at the age of 33, after finishing a charity run in Norfolk in 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giussani, D., Fletcher, A. & Gardner, D. Sex Differences in the Ovine Fetal Cortisol Response to Stress. Pediatr Res 69, 118–122 (2011). https://doi.org/10.1203/PDR.0b013e3182042a20
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3182042a20
This article is cited by
-
Fetal Sex Does Not Impact Placental Blood Flow or Placental Amino Acid Transfer in Late Gestation Pregnant Sheep With or Without Placental Insufficiency
Reproductive Sciences (2022)
-
Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics
Biology of Sex Differences (2013)