Abstract
Ligamentum flavum degeneration, including hypertrophy and ossification of the ligamentum flavum, leads to degenerative spinal stenosis in older adults. However, the underlying mechanisms of ligamentum flavum degeneration remain unclear, and therapeutic strategies are limited. Noncoding RNAs include microRNAs, circular RNAs, and long noncoding RNAs. As important epigenetic modifications, noncoding RNAs are involved in the progression of several age-related diseases, including ligamentum flavum degeneration. Previous studies have shown that noncoding RNAs can regulate the osteogenic differentiation and fibrosis of ligamentum flavum cells by regulating the expression of related genes. In this review, we discuss noncoding RNAs and their role in ligamentum flavum degeneration.
Similar content being viewed by others
Introduction
Degenerative spinal stenosis (DSS) is a very common condition in older adults and constitutes a heavy economic burden for both individuals and society1,2. DSS leads to numbness in the trunk and lower limbs, muscle weakness in the lower limbs, and even paraplegia2,3. Notably, several degenerative spinal structures can result in DSS, including intervertebral disc degeneration, spondylolysis, facet joint degeneration, and degeneration of ligamentum flavum (LF) tissues2,3. LF degeneration is a common cause of DSS. The LF, which is light yellow, connects the lamina of the vertebral bodies, covering the interlaminar space and forming the dorsal border of the spinal canal4. In a normal state, the LF is composed of approximately 70% elastin fibers and 30% collagen fibers organized in parallel layers, and its main function is to maintain the mechanical stability of the spine5. The two most common types of LF degeneration are hypertrophy of the ligamentum flavum (HLF) and ossification of the ligamentum flavum (OLF). LF degeneration in HLF is characterized by a loss of elastic fibers and increased number of collagen fibers, which further leads to fibrotic thickening and scar tissue formation. HLF is more common in the lumbar spine, and the hypertrophic LF compresses the spinal cord and nerve roots, eventually causing lumbar spinal stenosis4. The primary pathology of OLF is the ectopic ossification of LF tissues, which mainly occurs in the thoracic spine6,7. In OLF, the ossified LF narrows the thoracic spinal canal and compresses the spinal cord6,7. The mainstream therapy for HLF and OLF is surgical decompression because conservative treatment is usually ineffective2,3. However, surgical decompression for HLF and OLF is complicated and has a high risk of complications such as injury to the spinal cord or nerve roots, dural tears, and infection4,7. Therefore, there is an urgent need to explore the underlying mechanisms of LF degeneration to identify promising therapeutic targets and avoid surgical intervention.
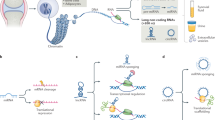
Epigenetic modifications refer to heritable and reversible changes in the function of a gene or genome without altering its DNA sequence8,9. Epigenetic modifications include but are not limited to posttranslational modifications, DNA methylation, histone modifications, and noncoding RNA (ncRNA) modulation9. Only 2% of transcripts in the human genome can be translated into proteins; however, most (98%) transcripts lack protein-coding ability and are termed ncRNAs10. ncRNAs include microRNAs (miRNAs), circular RNAs (circRNAs), and long noncoding RNAs (lncRNAs)10. ncRNAs were previously considered genetic “junk”; however, increasing evidence has demonstrated that ncRNAs are crucial in health maintenance and disease progression11. ncRNAs are involved in several degenerative musculoskeletal diseases, including osteoarthritis12 and intervertebral disc degeneration13. Notably, several ncRNAs are associated with the initiation and progression of LF degeneration14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. However, to our knowledge, no specialized systematic review has summarized the critical roles of ncRNAs in LF degeneration. Therefore, in this review, we systematically review the current literature to explore the biological functions of ncRNAs in LF degeneration in order to provide new insights to promote future research and clinical application of ncRNAs in LF degeneration (Fig. 1).
miRNAs in the degeneration of LF
miRNAs, which constitute the main group of small ncRNAs with average lengths of 19–25 nucleotides, are crucial in the posttranscriptional regulation of gene expression36,37. The core function of miRNAs is to induce the translational suppression or degradation of messenger RNAs (mRNAs) through binding to the 3′-untranslated regions (3′-UTRs) of targeted genes, which is called the competing endogenous RNA (ceRNA) mechanism36,37. Sixteen miRNAs, including eight HLF-related and eight OLF-related miRNAs, are associated with the progression of HLF and OLF (Table 1).
miRNAs in the HLF
miR-155
miR-155 has been previously reported to play important roles in several human diseases. For example, Wang et al. reported that high serum and tumor miR-155 levels are favorable prognostic markers for patients with breast cancer and that the overexpression of miR-155 can promote T-cell influx, delay tumor growth, and sensitize tumors to immune checkpoint blockade therapy in patients with breast cancer38. D’Adamo et al. reported that miR-155 suppressed autophagy in chondrocytes by regulating the expression of autophagy proteins, including ULK1, FOXO3, ATG14, ATG5, ATG3, GABARAPL1, and MAP11C339. Chen et al. reported that miR-155 expression was significantly increased in hypertrophic LF tissues compared with normal LF tissues (p < 0.01). Moreover, the authors also reported that the miR-155 expression level was positively associated with LF thickness (p < 0.01) and the protein levels of fibrosis-related genes (collagen I and III) (p < 0.01). Fibroblasts were also transfected with miR-155 mimics or sponge lentiviruses to further explore the biological functions of miR-155 in patients with HLF. The miR-155 mimic significantly increased the mRNA and protein levels of fibrosis-related genes (collagen I and III) in fibroblasts. Conversely, the miR-155 sponge reduced the mRNA and protein expression of fibrosis-related genes (collagen I and III) in fibroblasts. However, further studies on the regulation of miR-155 in patients with HLF have not yet been conducted14.
miR-21
miR-21 is thought to be involved in many human diseases, such as osteoarthritis40 and bone defects41. Zhang et al. reported that miR-21 promoted osteoarthritis by targeting GDF5 to decrease GDF5 expression levels and that the knockout of miR-21 could alleviate osteoarthritis in mice40. Qi et al. reported that a DNA tetrahedron could facilitate the repair of senescent bone defects by delivering miR-21 to regulate osteogenesis and angiogenesis41. Sun et al. explored the role of miR-21 in HLF pathogenesis because it has been reported to participate in the fibrosis of other organs42,43. The authors reported that miR-21 expression was significantly increased in hypertrophic LF tissues compared with control LF tissues. Moreover, miR-21 was distinctly associated with LF thickness and fibrosis score in patients with HLF. The overexpression of miR-21 increased the mRNA and protein levels of fibrosis-related genes (collagen I and III) and an inflammation-related gene (IL-6). The authors concluded that miR-21 elevates IL-6 expression, which leads to inflammation, fibrosis, and hypertrophy of LF tissues. However, they did not investigate the mechanism underlying the relationship between miR-21 and IL-6 expression19.
miR-221-3p
miR-221-3p has been reported to have several biological functions in human diseases, such as intervertebral disc degeneration44 and acute myeloid leukemia45. Zhang et al. suggested that miR-221-3p in small extracellular vesicles derived from M2 macrophages could inhibit pyroptosis in nucleus pulposus cells through the PTEN/NLRP3 signaling pathway44. Li et al. reported that small extracellular vesicles derived from acute myeloid leukemia cells could facilitate leukemogenesis by transferring miR-221-3p to reduce the expression level of GBP245. Xu et al. conducted a miRNA microarray analysis on samples from five patients with and without HLF to screen for potential miRNAs associated with HLF. Eighteen differentially expressed miRNAs were identified, including 15 upregulated (miR-23b, miR-21-5p, miR-202-3p, miR-155, miR-340-5p, miR-374a, miR-146b, miR-29c, miR-223, miR-486, miR-125a, miR-34a, miR-17, miR-148b, and miR-106b) and three downregulated miRNAs (miR-342-5p, miR-27a, and miR-221-3p). Xu et al. selected miR-221-3p from the miRNA microarray results for further investigation. The expression level of miR-221-3p was significantly lower in hypertrophic LF tissues than in normal LF tissues. miR-221-3p overexpression obviously decreased the mRNA levels of fibrosis-related genes (collagen I and III). Moreover, the authors reported that the downregulation of miR-221-3p contributed to HLF by directly targeting the 3’-UTR of TIMP2, a vital protein involved in the breakdown of the extracellular matrix16.
miR-10396b-3p
Li et al. performed miRNA sequencing analysis on 12 patients with and without HLF. Ninety-four differentially expressed miRNAs were identified, 35 of which were upregulated, and 59 of which were downregulated. The authors selected miR-10396b-3p from the miRNA sequencing analysis for further investigation. miR-10396b-3p expression was distinctly lower in hypertrophic LF tissues than in normal LF tissues. The authors reported that IL-11 is a downstream regulator of miR-10396b-3p and demonstrated that the overexpression of miR-10396b-3p could reduce the mRNA and protein levels of IL-11, which further decreased the expression of fibrotic markers (collagen I and III)25.
miR-4306
miR-4306 is a well-known miRNA involved in several diseases. Zhao et al. reported that miR-4306 affects the progression of triple-negative breast cancer by directly targeting SIX1/CDC42/VEGFA in triple-negative breast cancer46. Wang et al. reported that miR-4306 expression was distinctly decreased in nucleus pulposus tissues; further research indicated that miR-4306 regulated cell proliferation, extracellular matrix synthesis, and autophagy by directly targeting PAK647. Duan et al. reported that TCF7, a member of the T-cell factor/lymphoid enhancer factor transcription factor family, could interact with the SNAI2 promoter to activate the expression of SNAI2, which further promoted the hyperproliferation and fibrosis phenotype of LF cells. Moreover, the authors reported that the level of miR-4036 was significantly lower in hypertrophic LF tissues than in normal LF tissues and that the overexpression of miR-4306 promoted apoptosis but inhibited the viability and fibrosis of LF cells. Notably, the authors reported that miR-4036 was negatively regulated by SNAI2 and could reduce the expression of TCF7 by binding to the 3′-UTR of TCF7 mRNA. In vivo, experiments indicated that knockdown of TCF7 relieved hypertrophy and fibrosis of the LF in mice. The authors demonstrated that the TCF7/SNAI2/miR-4306 feedback loop is critical in HLF pathogenesis28.
miR-146a-5p and miR-221-3p
miR-146a-5p plays a variety of roles in human diseases. Yang et al. reported that in osteoarthritis, miR-146a-5p relieves the progression of osteoarthritis through the inhibition of SDF-1/CXCR4-induced autophagy in chondrocytes48. Rigg et al. reported that tumor-derived extracellular vesicles promoted melanoma brain metastasis via the transfer of miR-146a-5p to regulate the Notch pathway in astrocytes49. Ma et al. reported the downregulation of miR-146a-5p and miR-221-3p expression in hypertrophic LF tissues compared with normal LF tissues, and both were negatively correlated with increased LF thickness. The authors reported that umbilical cord mesenchymal stromal cell-derived extracellular vesicles rich in miR-146a-5p and miR-221-3p could relieve hypertrophic LF in in vivo and in vitro assays. Moreover, they demonstrated that miR-146a-5p and miR-221-3p could directly bind to the 3′-UTR regions of SMAD4 mRNA to decrease the SMAD4 expression level, which further blocked the TGF-β/SMAD4 signaling pathway to relieve fibrosis30.
miR-29a
miR-29a performs multiple biological functions in various diseases. miR-29a can regulate collagen synthesis, activate the NF-κB signaling pathway in tenocytes, and regulate macrophage polarization. Chen et al. reported that lipid nanoparticle-assisted miR-29a delivery via core-shell nanofibers is a promising treatment for tendon injury50. Zhao et al. reported that miR-29a inhibited breast tumor initiation by targeting KLF4 in cancer stem cells51. Wawrose et al. reported that the level of miR-29a was significantly lower in hypertrophic LF tissues than in normal LF tissues and that miR-29a expression was negatively associated with the mRNA level of collagen I. In in vitro experiments, miR-29a inhibition promoted collagen I mRNA expression. In contrast, the overexpression of miR-29a decreased collagen I mRNA expression. Therefore, the authors concluded that miR-29a is a negative regulator of the pathogenesis of HLF. However, the authors did not investigate the mechanisms underlying the role of miR-29a in the pathogenesis of HLF31.
miRNAs in patients with OLF
miR-342-3p
Dysregulated expression of miR-342-3p is involved in many human diseases. Xue et al. reported that miR-342-3p could suppress cell proliferation and migration by directly targeting AGR2 in non-small cell lung cancer52. Huang et al. reported that miR-342-3p promoted the osteogenic differentiation of umbilical cord mesenchymal stem cells by reducing SUFU expression53. Han et al. selected miR-342-3p on the basis of the results of miRNA sequencing analysis related to OLF21. The authors reported that miR-342-3p expression was significantly increased in ossified LF tissues compared with normal LF tissues. Further research revealed that miR-342-3p promoted the osteogenic differentiation of mesenchymal stem cells (MSCs) by directly targeting ATF3. Moreover, the authors reported that the overexpression of miR-342-3p was induced by the demethylation of the Enah/Vasp-Like CpG island33.
miR-132-3p
miR-132-3p has been reported to play vital roles in several human diseases, especially degenerative diseases. Hu et al. reported that miR-132-3p inhibits osteoblast differentiation by targeting EP300 and could serve as a promising target for the promotion of bone formation54. Zhao et al. reported that miR-132-5p could regulate the apoptosis and autophagy of SH-SY5Y cells by decreasing the expression of the target gene ULK1 and that miR-132-5p might be a prospective therapeutic target for Parkinson’s disease55. Qu et al. reported that the level of miR-132-3p decreased during the osteogenic differentiation of LF cells. Therefore, inhibiting miR-132-3p promoted the osteogenesis of LF cells; however, the overexpression of miR-132-3p reduced the osteogenesis of LF cells. Furthermore, bioinformatics analysis revealed that FOXO1, GDF5, and SOX6 are potential downstream factors of miR-132-3p, and the authors demonstrated that miR-132-3p inhibited the osteogenesis of LF cells by directly binding to the 3′-UTRs of FOXO1, GDF5 and SOX615.
miR-199b-5p
miR-199b-5p is an important epigenetic regulator in the pathogenesis of human diseases. Ruan et al. reported that breast cancer cell-secreted miR-199b-5p promoted brain metastasis by targeting solute carrier transporters to hijack neuron‒astrocyte metabolic coupling56. Feng et al. reported that miR-199b-5p was significantly increased during the progression of osteoarthritis and that a reduction in miR-199b-5p could relieve the pathological changes in chondrocytes by targeting FZD6 and GCNT257. Qu et al. selected miR-199b-5p for further investigation because it is reportedly critical in the ossification of the posterior longitudinal ligament and bone marrow stromal cells58,59. The authors reported that the expression of miR-199b-5p was significantly decreased during the osteogenesis of LF cells and that the overexpression of miR-199b-5p inhibited the osteogenic differentiation of LF cells. Bioinformatics analysis revealed that miR-199b-5p can directly bind to the 3′-UTR of JAG1, which is a vital Notch ligand and is vital in the Notch signaling pathway60. The Notch signaling pathway is crucial for osteogenic differentiation, bone healing, and skeletal development61. The authors demonstrated that the overexpression of miR-199b-5p inhibited the osteogenic differentiation of LF cells by targeting JAG1 and modulating the Notch signaling pathway18.
miR-615-3p
miR-615-3p has multiple biological functions in the pathological and physiological processes of human diseases. For example, miR-199b-5p promoted the chondrogenic differentiation of C3H10T1/2 cells by directly targeting JAG162. In addition, Lin et al. reported that miR-199b-5p could suppress tumor angiogenesis mediated by vascular endothelial cells by reducing ALK1 expression in breast cancer63. Yin et al. reported that miR-615-3p was downregulated during the osteogenic differentiation of human osteoblasts, MSCs, and LF cells. In vitro experiments revealed that the overexpression of miR-615-3p inhibited the osteogenic differentiation of LF cells, whereas its knockdown promoted osteogenic differentiation. Moreover, the authors demonstrated that miR-615-3p regulated the osteogenic differentiation of LF cells by directly targeting the 3′-UTRs of FOXO1 and GDF520.
miR-182
A variety of studies have explored the important roles of miR-182 in human diseases. Yang et al. reported that miR-182 regulated macrophage polarization and redox reactions to promote repair after ischemic cardiac injury by targeting RASA164. Long et al. reported that exosomal miR-182 derived from bone marrow mesenchymal stem cells contributed to the carfilzomib resistance of multiple myeloma cells by targeting SOX665. Zhang et al. reported that miR-182 expression was significantly decreased, but NAMPT expression was markedly increased in ossified LF tissues compared with normal LF tissues. Furthermore, the authors demonstrated that the overexpression of miR-182 or the inhibition of NAMPT reduced the osteogenic differentiation of LF cells. Notably, bioinformatics analysis and in vitro experiments demonstrated that miR-182 regulated the osteogenic differentiation of LF cells by directly targeting the 3′-UTR of NAMPT mRNA to decrease NAMPT expression22.
miR-29a-5p
The role of miR-29a-5p in multiple diseases has been widely investigated. Dai et al. reported that miR-29a-5p is a tumor-suppressive miRNA in gliomas and that miR-29a-5p suppresses the proliferation, invasion, and migration of gliomas by directly binding to DHRS466. Wang et al. reported that parathyroid hormone-induced endothelial-to-mesenchymal transition through the miR-29a-5p/GSAP/Notch1 pathway, which further promoted valvular calcification in nonchronic kidney disease67. In a study by Feng et al., miR-29a-5p expression was downregulated in ossified LF tissues, whereas SATB2 expression was increased compared with that in normal LF tissues. The authors observed reduced miR-29a-5p expression during the osteogenic differentiation of LF cells, and the overexpression of miR-29a-5p reduced the osteogenic differentiation of LF cells by directly binding to the 3’-UTR of SATB2. Moreover, they reported that SATB2 knockdown significantly reduced SIRT1 levels and Smad3 acetylation. The authors also showed that miR-29a-5p regulated the osteogenic differentiation of LF cells through the SATB2/SIRT1/SMAD3 deacetylation pathway23.
miR-19b-3p
miR-19b-3p has attracted increasing research attention because of its important biological functions in the maintenance of health. Zhang et al. developed a biomimetic composite hydrogel to facilitate new bone formation via the transfer of miR-19b-3p to regulate WWP1 expression68. Chen et al. reported that tumor-derived exosomal miR-19b-3p promoted M2 macrophage polarization by targeting PTPRD, which further inhibited PTPRD-mediated dephosphorylation of STAT3 to promote lung adenocarcinoma metastasis69. Han et al. conducted transcriptome sequencing, including miRNA sequencing, on four ossified and four normal LF tissues to explore the changing expression profiles of OLF. The results revealed 28 differentially expressed miRNAs (fold change > 2, p < 0.05), including 12 upregulated and 16 downregulated miRNAs. The authors selected miR-19b-3p, a downregulated miRNA identified via miRNA sequencing, for further analyses. They reported that miR-19b-3p was downregulated in ossified LF tissues and human MSCs following osteogenic induction. Therefore, the overexpression of miR-19b-3p decreased the osteogenic differentiation of LF cells. However, the underlying mechanisms have not yet been investigated21.
miR-3064-5p
miR-3064-5p has been reported to participate in several diseases, such as metainflammation. Luo et al. reported that macrophage exosomes regulate palmitic acid-induced inflammation through the transfer of miR-3064-5p to activate NF-κB signaling by targeting IκBα70. Huang et al. reported that, compared with that in normal LF tissues, miR-3064-5p was decreased in ossified LF tissues and gradually decreased in a time-dependent manner during osteogenic differentiation. The overexpression of miR-3064-5p significantly inhibited the osteogenic differentiation of MSCs. MEOX1 is a transcription factor associated with somitic compartment gene expression, embryonic development, and cancer progression71. The authors reported that MEOX1 expression was significantly increased in ossified LF tissues and during the osteogenic differentiation of human MSCs. On the basis of bioinformatic analysis and subsequent experimental validation, the authors reported that miR-3064-5p regulated the osteogenic differentiation of MSCs by directly targeting the 3′-UTR of MEOX134.
Circular RNAs in LF degeneration
CircRNAs are characterized by a single-stranded covalent closed-loop structure without a 5′ cap structure and a 3′ polyadenylated tail72. Notably, many circRNAs have been identified through the development of high-throughput RNA sequencing technologies72. In recent years, increasing evidence has shown that circRNAs play critical roles in human health and disease by sponging miRNAs or proteins, serving as scaffolds for protein complex formation, and translating them into peptides or proteins73,74,75. CircRNAs have been shown to participate in the pathogenesis of several degenerative diseases of the bone and joints; however, their role in LF degeneration has not been fully reviewed76,77. Two circRNAs have been reported to be associated with HLF in LF degeneration, and one circRNA has been reported to contribute to OLF (Table 2).
Circular RNAs in patients with HLF
hsa_circ_0052318
Chen et al. performed circRNA sequencing analysis on three hypertrophic and three normal LF tissues. HLF tissues were collected from patients with LF hypertrophy at the L4/L5 level, whereas non-HLF tissues were collected from the same patients at the L3/L4 level without evidence of HLF. In total, 2439 differentially expressed circRNAs (fold change ≥ 2, p < 0.05) were identified through sequencing analysis. Among the 2439 differentially expressed circRNAs, 1276 were upregulated, and 1163 were downregulated. The authors selected hsa_circ_0052318 for further analysis because it was the largest node in the circRNA‒miRNA coexpression network. In cellular experiments, the authors discovered that the overexpression of hsa_circ_0052318 significantly increased the mRNA and protein levels of collagen I and III. However, the underlying mechanisms have not yet been explored24.
hsa_circ_0057105
hsa_circ_0057105 is derived from exons 7 to 11 of the PDK1 gene on chromosome 2. Cen et al. reported that hsa_circ_0057105 balanced epithelial‐mesenchymal transition and ferroptosis vulnerability in renal cell carcinoma by sponging miR-577 to regulate COL1A1 and VDAC2 expression78. Zhang et al. performed high-throughput second-generation sequencing analysis on five hypertrophic and five normal LF tissues and identified 107 differentially expressed circRNAs, of which 67 were upregulated and 40 were downregulated in hypertrophic tissues compared with normal LF tissues (p < 0.05). The authors selected hsa_circ_0057105 for further analysis because it was the most significantly different among the 107 differentially expressed circRNAs. The authors demonstrated that hsa_circ_0057105 expression increased in hypertrophic LF tissues, and cell experiments revealed that hsa_circ_0057105 promoted the viability, proliferation, migration, and fibrosis of LF cells. The authors reported that miR-4731-5p was a potential target of hsa_circ_0057105 and that miR-4731-5p overexpression suppressed the viability, proliferation, migration, and fibrosis of LF cells. TNXB was previously reported to be involved in the osteogenic differentiation of bone marrow MSCs79, which was significantly greater in ossified LF tissues than in normal LF tissues. The authors demonstrated that hsa_circ_0057105 promoted the viability, proliferation, migration, and fibrosis of LF cells by increasing TNXB expression by sponging miR-4731. In vivo experiments revealed that miR-4731 knockdown significantly induced fibrosis in LF tissues, whereas the opposite results were observed with hsa_circ_0057105 silencing; moreover, the effect of hsa_circ_0057105 silencing could be partly reversed by miR-4731 knockdown32.
Circular RNAs in patients with OLF
hsa_circ_0050139
Han et al. conducted microarray analyses of ncRNAs to explore their potential roles in patients with OLF. In their report, 627 differentially expressed circRNAs were identified with a standard “fold change > 2 and p < 0.05”, including 244 upregulated circRNAs and 383 downregulated circRNAs in OLF tissues. Han et al. further validated the role of hsa_circ_0050139 in OLF pathogenesis. They discovered that hsa_circ_0050139 was significantly increased during the osteogenic induction of LF cells and human MSCs. Moreover, osteogenic biomarkers and alkaline phosphatase staining were distinctly decreased after hsa_circ_0050139 was silenced in human MSCs21.
lncRNAs in the degeneration of LF
lncRNAs are a class of transcripts with lengths > 200 nucleotides80. Generally, lncRNAs are classified into six categories: sense, antisense, intergenic, intron, bidirectional, and enhancer lncRNAs80. lncRNAs can perform biological functions through various mechanisms, such as the regulation of gene expression, chromatin modification, and genomic imprinting. Like circRNAs, the most common biological mechanism of lncRNAs is to regulate the mRNA levels of genes by sponging targeted miRNAs, which is also called the ceRNA hypothesis80. Previous studies have demonstrated that lncRNAs are crucial in patients with orthopedic diseases. Five lncRNAs are involved in LF degeneration, including one associated with HLF and four associated with OLF (Table 2).
lncRNAs in patients with HLF
lncRNA X-inactive specific transcript (XIST)
lncRNA XIST, a well-known lncRNA, has been reported to play important roles in several diseases, such as osteoporosis81 and multiple cancers82,83. Chen et al. reported that lncRNA XIST inhibited osteoblast differentiation and aggravated osteoporosis through Nrf2 hyperactivation by regulating CUL381. Ma et al. reported that lncRNA XIST regulated breast cancer stem cells via the IL-6/STAT3 signaling pathway83. With respect to HLF, Cao et al. conducted RNA sequencing on three HLF and three non-HLF tissues and selected lncRNA XIST for further research because it presented the most significant difference in the hub mRNA‒miRNA-lncRNA ceRNA network. In vitro experiments revealed that lncRNA XIST promoted the proliferation, apoptosis, and fibrosis of LF cells by activating autophagy. The authors demonstrated that lncRNA XIST might promote the pathological progression of LF cells by regulating VEGFA expression by sponging miR‑302b‑3p. In vivo, experiments further confirmed that lncRNA XIST knockdown significantly relieved hypertrophy and fibrosis in LF29.
lncRNAs in patients with OLF
lncRNA ENST00000608133 and lncRNA ENST00000599584
Han et al. determined the expression profiles of lncRNAs during OLF pathogenesis. In total, 2567 lncRNAs were differentially expressed in OLF tissues (fold change > 2, P < 0.05), of which 1817 were upregulated and 750 were downregulated. The authors selected two upregulated lncRNAs (ENST00000608133 and ENST00000599584) for further experiments and demonstrated that the knockdown of ENST00000608133 or ENST00000599584 reduced the osteogenic capacity of human MSCs21.
lncRNA HOX antisense intergenic RNA myeloid 1 (HOTAIRM1)
Previous studies have shown that lncRNA HOTAIRM1 plays important role in several human diseases. For example, Guo et al. reported that exosome-encapsulated lncRNA HOTAIRM1 promoted airway remodeling in chronic obstructive pulmonary disease by promoting myofibroblast differentiation84. Chao et al. reported that lncRNA HOTAIRM1 inhibited cell proliferation and invasion by sponging miR-106a-5p to increase ARHGAP24 expression in ovarian cancer85. A study by Ren et al. revealed that lncRNA HOTAIRM1 was upregulated in ossified LF tissues compared with normal LF tissues, according to the GSE106253 microarray data. The authors demonstrated that the expression of lncRNA HOTAIRM1 was increased during osteogenesis in human MSCs and in hFOB1.19, C3H/10T1/2, and MC3T3-E1 cells. Notably, the authors reported that HOTAIRM1 was downregulated during RANKL-induced osteoclast differentiation. Using in vitro experiments, the authors reported that the knockdown of lncRNA HOTAIRM1 inhibited osteoclast differentiation but promoted osteoclast differentiation. The authors demonstrated that lncRNA HOTAIRM1 inhibited RANKL-induced osteoclastogenesis through the NF-κB pathway35.
lncRNA DLX6 Antisense RNA 14 (DLX6-AS1)
lncRNA DLX6-AS1 performs important biological functions in human diseases. For example, Wang et al. reported that lncRNA DLX6-AS1 promoted myocardial ischemia-reperfusion injury via the miR-204-5p/FBXW7 axis86. Guo et al. reported that lncRNA DLX6-AS1 promoted cell proliferation, invasion, migration, and epithelial-to-mesenchymal transition by modulating the Wnt/β-catenin signaling pathway in bladder cancer87. Zhang et al. reported that the lncRNA DLX6-AS1, also known as lnc-EVF2, was expressed at higher levels in LF samples than in normal LF tissues, according to the microarray data of the GSE106253 dataset. The authors also reported that lncRNA DLX6-AS1 was gradually upregulated during osteogenic induction at various time points in MC3T3-E1, C3H10T1/2, and mouse MSCs. They demonstrated that the overexpression of lncRNA DLX6-AS1 promoted osteogenic differentiation by regulating the Notch signaling pathway27.
In conclusion, DSS due to LF degeneration has become the main contributor to low back pain, which places a heavy economic burden on society and individuals2,3. However, there are currently no reliable biomarkers for predicting or treating LF degeneration. As critical components of epigenetic modifications, ncRNAs, including miRNAs, circRNAs, and lncRNAs, are crucial in the onset and development of LF degeneration. However, there is still a long way to go before clinical translation can be achieved for ncRNAs in LF degeneration. First, while all of these ncRNAs have been proven to be effective in cell or animal experiments, none have been verified in humans. Second, more detailed and accurate mechanisms of these ncRNAs in LF degeneration have not been reported, such as why a given ncRNA is upregulated or downregulated. Third, the expression levels of these ncRNAs have not been assessed in the blood samples of patients, which limits the application of ncRNAs as diagnostic markers or monitoring indicators for LF degeneration. Fourth, ncRNAs can perform their biological functions through several molecular mechanisms, such as serving as protein scaffolds and translating them into peptides. However, all currently published studies have focused only on the ceRNA mechanism of ncRNAs in LF degeneration. Therefore, future studies should be conducted to address these concerns. These efforts will benefit the management of patients with LF degeneration through the application of more accurate, effective, and tailored ncRNA therapies.
References
Austevoll, I. M. et al. Decompression with or without fusion in degenerative lumbar spondylolisthesis. N. Engl. J. Med. 385, 526–538 (2021).
Katz, J. N., Zimmerman, Z. E., Mass, H. & Makhni, M. C. Diagnosis and management of lumbar spinal stenosis: a review. Jama 327, 1688–1699 (2022).
Chen, Z. Q. & Sun, C. G. Clinical guideline for treatment of symptomatic thoracic spinal stenosis. Orthop. Surg. 7, 208–212 (2015).
Byvaltsev, V. A. et al. Molecular and genetic mechanisms of spinal stenosis formation: systematic review. Int. J. Mol. Sci. 23, 13479 (2022).
Shin, H. K., Seo, K. J., Lee, J. Y., Jeon, S. R. & Yune, T. Y. GSK-3β and β-catenin signaling pathway is involved in myofibroblast transition of ligamentum flavum in lumbar spinal stenosis patients. Spine 48, 1472–1479 (2023).
Zhao, Y., Xiang, Q., Lin, J., Jiang, S. & Li, W. High body mass index is associated with an increased risk of the onset and severity of ossification of spinal ligaments. Front. Surg. 9, 941672 (2022).
Zhao, Y., Xiang, Q., Lin, J., Jiang, S. & Li, W. High systemic immune-inflammation index and body mass index are independent risk factors of the thoracic ossification of the ligamentum flavum. Mediators Inflamm. 2022, 4300894 (2022).
Okada, Y. & Yamaguchi, K. Epigenetic modifications and reprogramming in paternal pronucleus: sperm, preimplantation embryo, and beyond. Cell. Mol. life Sci. CMLS 74, 1957–1967 (2017).
Xiang, Q., Zhao, Y., Lin, J., Jiang, S. & Li, W. Epigenetic modifications in spinal ligament aging. Ageing Res. Rev. 77, 101598 (2022).
Cech, T. R. & Steitz, J. A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157, 77–94 (2014).
The expanding world of noncoding RNA biology. Nat. Cell Biol. 24, 1447 (2022).
Zhang, Z., Yang, P., Wang, C. & Tian, R. LncRNA CRNDE hinders the progression of osteoarthritis by epigenetic regulation of DACT1. Cell. Mol. life Sci. CMLS 79, 405 (2022).
Chen, X. et al. C-type lectin domain-containing protein CLEC3A regulates proliferation, regeneration and maintenance of nucleus pulposus cells. Cell. Mol. life Sci. CMLS 79, 435 (2022).
Chen, J. et al. Hypertrophy of ligamentum flavum in lumbar spine stenosis is associated with increased miR-155 level. Dis. markers 2014, 786543 (2014).
Qu, X., Chen, Z., Fan, D., Sun, C. & Zeng, Y. MiR-132-3p regulates the osteogenic differentiation of thoracic ligamentum flavum cells by inhibiting multiple osteogenesis-related genes. Int. J. Mol. Sci. 17, 1370 (2016).
Xu, Y. Q., Zhang, Z. H., Zheng, Y. F. & Feng, S. Q. MicroRNA-221 regulates hypertrophy of ligamentum flavum in lumbar spinal stenosis by targeting TIMP-2. Spine 41, 275–282 (2016).
Mori, T. et al. MicroRNA transcriptome analysis on hypertrophy of ligamentum flavum in patients with lumbar spinal stenosis. Spine Surg. Relat. Res. 1, 211–217 (2017).
Qu, X. et al. MiR-199b-5p inhibits osteogenic differentiation in ligamentum flavum cells by targeting JAG1 and modulating the Notch signalling pathway. J. Cell. Mol. Med. 21, 1159–1170 (2017).
Sun, C., Tian, J., Liu, X. & Guan, G. MiR-21 promotes fibrosis and hypertrophy of ligamentum flavum in lumbar spinal canal stenosis by activating IL-6 expression. Biochem. Biophys. Res. Commun. 490, 1106–1111 (2017).
Yin, J. et al. MiR-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1. Cell Biol. Int. 41, 779–786 (2017).
Han, Y. et al. A transcriptome-level study identifies changing expression profiles for ossification of the ligamentum flavum of the spine. Mol. Ther. Nucleic acids 12, 872–883 (2018).
Zhang, Q. et al. Overexpression of miR-182 inhibits ossification of ligamentum flavum cells by targeting NAMPT. Exp. Cell Res. 367, 119–131 (2018).
Feng, F. et al. miR-29a-5p Ttargets SATB2 and regulates the SIRT1/Smad3 deacetylation pathway to inhibit thoracic ligamentum flavum cell osteogenesis. Spine 45, E1057–E1065 (2020).
Chen, J. et al. Circular RNA expression profile in patients with lumbar spinal stenosis associated with hypertrophied ligamentum flavum. Spine 46, E916–E925 (2021).
Li, P. et al. miR-10396b-3p inhibits mechanical stress-induced ligamentum flavum hypertrophy by targeting IL-11. FASEB J. 35, e21676 (2021).
Zhang, B. et al. Dysregulation of MicroRNAs in hypertrophy and ossification of ligamentum flavum: new advances, challenges, and potential directions. Front. Genet. 12, 641575 (2021).
Zhang, Z. et al. Preosteoblast-enriched lnc-Evf2 facilitates osteogenic differentiation by targeting Notch. Acta Biochim. et Biophys. Sin. 53, 179–188 (2021).
Duan, Y., Li, J., Qiu, S., Ni, S. & Cao, Y. TCF7/SNAI2/miR-4306 feedback loop promotes hypertrophy of ligamentum flavum. J. Transl. Med. 20, 468 (2022).
Cao, Y., Li, J., Qiu, S., Ni, S. & Duan, Y. LncRNA XIST facilitates hypertrophy of ligamentum flavum by activating VEGFA-mediated autophagy through sponging miR-302b-3p. Biol. direct 18, 25 (2023).
Ma, C. et al. Amelioration of ligamentum flavum hypertrophy using umbilical cord mesenchymal stromal cell-derived extracellular vesicles. Bioact. Mater. 19, 139–154 (2023).
Wawrose, R. A. et al. MicroRNA-29a: a novel target for non-operative management of symptomatic lumbar spinal stenosis. Eur. Spine J. 33, 892-899 (2023).
Zhang, K. et al. Circular RNA PDK1 targets miR-4731-5p to enhance TNXB expression in ligamentum flavum hypertrophy. FASEB J. 37, e22877 (2023).
Han, Y. et al. miR-342-3p promotes osteogenic differentiation via targeting ATF3. FEBS Lett. 592, 4051–4065 (2018).
Huang, M., Li, X. & Li, G. Mesenchyme homeobox 1 mediated-promotion of osteoblastic differentiation is negatively regulated by mir-3064-5p. Differ. Res. Biol. Divers. 120, 19–27 (2021).
Ren, Y. et al. HOTAIRM1 promotes osteogenic differentiation and alleviates osteoclast differentiation by inactivating the NF-κB pathway. Acta Biochim. et. Biophys. Sin. 53, 201–211 (2021).
Nelson, P., Kiriakidou, M., Sharma, A., Maniataki, E. & Mourelatos, Z. The microRNA world: small is mighty. Trends Biochem. Sci. 28, 534–540 (2003).
Zhao, Y. & Srivastava, D. A developmental view of microRNA function. Trends Biochem. Sci. 32, 189–197 (2007).
Wang, J. et al. Breast cancer cell-derived microRNA-155 suppresses tumor progression via enhancing immune cell recruitment and antitumor function. J. Clin. Investig. 132, e157248 (2022).
D’Adamo, S., Alvarez-Garcia, O., Muramatsu, Y., Flamigni, F. & Lotz, M. K. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr. Cartil. 24, 1082–1091 (2016).
Zhang, A. et al. Knockout of miR-21-5p alleviates cartilage matrix degradation by targeting Gdf5 in temporomandibular joint osteoarthritis. Bone Jt. Res. 9, 689–700 (2020).
Qi, L. et al. DNA tetrahedron delivering miR-21-5p promotes senescent bone defects repair through synergistic regulation of osteogenesis and angiogenesis. Adv. Healthc. Mater. e2401275 (2024).
Lu, Y. et al. METTL3-mediated m6A RNA methylation induces the differentiation of lung resident mesenchymal stem cells into myofibroblasts via the miR-21/PTEN pathway. Respir. Res. 24, 300 (2023).
Huang, Y. et al. Isovitexin alleviates hepatic fibrosis by regulating miR-21-mediated PI3K/Akt signaling and glutathione metabolic pathway: based on transcriptomics and metabolomics. Phytomedicine 121, 155117 (2023).
Zhang, K. et al. M2 macrophage-derived small extracellular vesicles ameliorate pyroptosis and intervertebral disc degeneration. Biomater. Res. 28, 0047 (2024).
Li, M. et al. Small extracellular vesicles derived from acute myeloid leukemia cells promote leukemogenesis by transferring miR-221-3p. Haematologica 109, 3209–3221 (2024).
Zhao, Z. et al. Transcriptional downregulation of miR-4306 serves as a new therapeutic target for triple negative breast cancer. Theranostics 9, 1401–1416 (2019).
Wang, D. et al. The role of the miR-4306/PAK6 axis in degenerative nucleus pulposus cells in human intervertebral disc degeneration. Cell. Signal. 102, 110528 (2023).
Yang, T. et al. MicroRNA-146a-5p alleviates the pathogenesis of osteoarthritis by inhibiting SDF-1/CXCR4-induced chondrocyte autophagy. Int. Immunopharmacol. 117, 109938 (2023).
Rigg, E. et al. Inhibition of extracellular vesicle-derived miR-146a-5p decreases progression of melanoma brain metastasis via Notch pathway dysregulation in astrocytes. J. Extracell. Vesicles 12, e12363 (2023).
Chen, W. et al. Lipid nanoparticle-assisted miR29a delivery based on core-shell nanofibers improves tendon healing by cross-regulation of the immune response and matrix remodeling. Biomaterials 291, 121888 (2022).
Zhao, Q. et al. miR-29a-KLF4 signaling inhibits breast tumor initiation by regulating cancer stem cells. Int. Immunopharmacol. 130, 111797 (2024).
Xue, X. et al. miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett. 412, 170–178 (2018).
Huang, M., Qing, Y., Shi, Q., Cao, Y. & Song, K. miR-342-3p elevates osteogenic differentiation of umbilical cord mesenchymal stem cells via inhibiting Sufu in vitro. Biochem. Biophys. Res. Commun. 491, 571–577 (2017).
Hu, Z. et al. miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci. Rep. 5, 18655 (2015).
Zhao, J., Yang, M., Li, Q., Pei, X. & Zhu, X. miR-132-5p regulates apoptosis and autophagy in MPTP model of Parkinson’s disease by targeting ULK1. Neuroreport 31, 959–965 (2020).
Ruan, X. et al. Breast cancer cell-secreted miR-199b-5p hijacks neurometabolic coupling to promote brain metastasis. Nat. Commun. 15, 4549 (2024).
Feng, T. et al. Inhibition of miR-199b-5p reduces pathological alterations in osteoarthritis by potentially targeting Fzd6 and Gcnt2. eLife 12, RP92645 (2024).
Xu, G. et al. Integrated miRNA-mRNA network revealing the key molecular characteristics of ossification of the posterior longitudinal ligament. Medicine 99, e20268 (2020).
Zhao, R. et al. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3β/β-catenin signaling pathway. Biochemical Biophys. Res. Commun. 477, 749–754 (2016).
Zhao, C. et al. Regenerative failure of intrahepatic biliary cells in Alagille syndrome rescued by elevated Jagged/Notch/Sox9 signaling. Proc. Natl Acad. Sci. USA 119, e2201097119 (2022).
Pakvasa, M. et al. Notch signaling: Its essential roles in bone and craniofacial development. Genes Dis. 8, 8–24 (2021).
Zhang, M. et al. miR-199b-5p promoted chondrogenic differentiation of C3H10T1/2 cells by regulating JAG1. J. Tissue Eng. Regen. Med. 14, 1618–1629 (2020).
Lin, X. et al. MiR-199b-5p suppresses tumor angiogenesis mediated by vascular endothelial cells in breast cancer by targeting ALK1. Front. Genet. 10, 1397 (2019).
Yang, Y. et al. miR-182/183-Rasa1 axis induced macrophage polarization and redox regulation promotes repair after ischemic cardiac injury. Redox Biol. 67, 102909 (2023).
Long, S. et al. Exosomal miR-182 derived from bone marrow mesenchymal stem cells drives carfilzomib resistance of multiple myeloma cells by targeting SOX6. J. Orthop. Surg. Res. 18, 937 (2023).
Dai, Y. et al. miR-29a-5p regulates the proliferation, invasion, and migration of gliomas by targeting DHRS4. Front. Oncol. 10, 1772 (2020).
Wang, L. et al. PTH-induced EndMT via miR-29a-5p/GSAP/Notch1 pathway contributed to valvular calcification in rats with CKD. Cell Prolif. 54, e13018 (2021).
Guo, R., Wu, C., Liu, F., Dong, T. & Zhang, T. Biomimetic composite hydrogel promotes new bone formation in rat bone defects through regulation of miR-19b-3p/WWP1 axis by loaded extracellular vesicles. J. Nanobiotechnol. 21, 459 (2023).
Chen, J. et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 11, e478 (2021).
Luo, H. et al. Macrophage exosomes mediate palmitic acid-induced metainflammation by transferring miR-3064-5p to target IκBα and activate NF-κB signaling. J. Adv. Res. S2090-1232(24)00261-3 (2024).
Zeng, G. et al. The role of MEOX1 in non-neoplastic and neoplastic diseases. Biomed. Pharmacother. 158, 114068 (2023).
Liu, C. X. & Chen, L. L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Li, X., Yang, L. & Chen, L. L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell 71, 428–442 (2018).
Chen, K. et al. CircFam190a: a critical positive regulator of osteoclast differentiation via enhancement of the AKT1/HSP90β complex. Exp. Mol. Med. 55, 2051–2066 (2023).
Yu, J. et al. Circular RNA cFAM210A, degradable by HBx, inhibits HCC tumorigenesis by suppressing YBX1 transactivation. Exp. Mol. Med. 55, 2390–2401 (2023).
Hu, L., Wu, W. & Zou, J. Circular RNAs: typical biomarkers for bone-related diseases. J. Zhejiang Univ. Sci. B 23, 975–988 (2022).
Wang, J. et al. Oxidative stress-induced circKIF18A downregulation impairs MCM7-mediated anti-senescence in intervertebral disc degeneration. Exp. Mol. Med. 54, 285–297 (2022).
Cen, J. et al. Hsa_circ_0057105 modulates a balance of epithelial-mesenchymal transition and ferroptosis vulnerability in renal cell carcinoma. Clin. Transl. Med. 13, e1339 (2023).
Otabe, K. et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res. 33, 1–8 (2015).
Chen, L. L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 41, 761–772 (2016).
Chen, X., Ma, F., Zhai, N., Gao, F. & Cao, G. Long non‑coding RNA XIST inhibits osteoblast differentiation and promotes osteoporosis via Nrf2 hyperactivation by targeting CUL3. Int. J. Mol. Med. 48, 137 (2021).
Yang, J., Qi, M., Fei, X., Wang, X. & Wang, K. Long non-coding RNA XIST: a novel oncogene in multiple cancers. Mol. Med. 27, 159 (2021).
Ma, Y. et al. LncRNA XIST regulates breast cancer stem cells by activating proinflammatory IL-6/STAT3 signaling. Oncogene 42, 1419–1437 (2023).
Guo, H. et al. Exosome-encapsulated lncRNA HOTAIRM1 contributes to PM(2.5)-aggravated COPD airway remodeling by enhancing myofibroblast differentiation. Sci. China Life Sci. 67, 970–985 (2024).
Chao, H., Zhang, M., Hou, H., Zhang, Z. & Li, N. HOTAIRM1 suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 243, 117296 (2020).
Wang, F. & Wu, Y. lncRNA DLX6-AS1 promotes myocardial ischemia-reperfusion injury through mediating the miR-204-5p/FBXW7 Axis. Mediators Inflamm. 2023, 9380398 (2023).
Guo, J. et al. The lncRNA DLX6-AS1 promoted cell proliferation, invasion, migration and epithelial-to-mesenchymal transition in bladder cancer via modulating Wnt/β-catenin signaling pathway. Cancer Cell Int. 19, 312 (2019).
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82172480).
Author information
Authors and Affiliations
Contributions
Yongzhao Zhao: conceptualization, data curation, writing - original draft; Qian Xiang: software, investigation, data curation; Shuo Tian: writing - review & editing, visualization; Zhenquan Wu: formal analysis, writing - original draft; Jialiang Lin: resources; Longjie Wang: software; Zhuoran Sun: supervision; Weishi Li: project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Xiang, Q., Tian, S. et al. Noncoding RNA as a crucial epigenetic modulator in the degeneration of the ligamentum flavum. Exp Mol Med 56, 2551–2558 (2024). https://doi.org/10.1038/s12276-024-01348-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s12276-024-01348-2